| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-30 21:03:45 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003514 |
|---|
| Identification |
|---|
| Common Name | Brompheniramine |
|---|
| Class | Small Molecule |
|---|
| Description | Histamine H1 antagonist used in treatment of allergies, rhinitis, and urticaria. [PubChem] |
|---|
| Contaminant Sources | - FooDB Chemicals
- STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Anti-Allergic Agent
- Bromide Compound
- Drug
- Food Toxin
- Histamine H1 Antagonist
- Organic Compound
- Organobromide
- Synthetic Compound
|
|---|
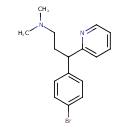
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(p-Bromophenyl)-1-(2-pyridyl)-3-dimethylaminopropane | ChEBI | | 2-(p-Bromo-alpha-(2-dimethylaminoethyl)benzyl)pyridine | ChEBI | | 3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine | ChEBI | | 3-(p-Bromophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine | ChEBI | | [3-(4-Bromo-phenyl)-3-pyridin-2-yl-propyl]-dimethyl-amine | ChEBI | | Bromfeniramina | ChEBI | | Brompheniraminum | ChEBI | | Brotane | Kegg | | 2-(p-Bromo-a-(2-dimethylaminoethyl)benzyl)pyridine | Generator | | 2-(p-Bromo-α-(2-dimethylaminoethyl)benzyl)pyridine | Generator | | Antihistamine compound | HMDB | | Brompheniramine maleate | HMDB, MeSH | | D-Brompheniramine | HMDB | | Dexbromfeniramina | HMDB | | Dexbrompheniramine | HMDB | | Dexbrompheniramine brompheniramine | HMDB | | Dexbrompheniramine maleate | HMDB | | Dexbrompheniramine maleate salt | HMDB | | Dexbrompheniraminum | HMDB | | Dimetane | HMDB, MeSH | | Dimetane-ten | HMDB, MeSH | | Dimetapp allergy | HMDB, MeSH | | Ilvin | HMDB | | Parabromdylamine | HMDB | | Parabromodylamine | HMDB | | Veltane | HMDB | | Dimetane ten | MeSH, HMDB | | Maleate, brompheniramine | MeSH, HMDB | | Whitehall-robins brand OF brompheniramine maleate | MeSH, HMDB | | Para bromdylamine | MeSH, HMDB | | Chlorphed | MeSH, HMDB | | Brompheniramine maleate (1:1) | MeSH, HMDB | | Oraminic 2 | MeSH, HMDB | | Oraminic-2 | MeSH, HMDB | | Roberts brand OF brompheniramine maleate | MeSH, HMDB | | Robins brand OF brompheniramine maleate | MeSH, HMDB | | P Bromdylamine | MeSH, HMDB | | P-Bromdylamine | MeSH, HMDB | | Vortech brand OF brompheniramine maleate | MeSH, HMDB | | Para-bromdylamine | MeSH, HMDB |
|
|---|
| Chemical Formula | C16H19BrN2 |
|---|
| Average Molecular Mass | 319.239 g/mol |
|---|
| Monoisotopic Mass | 318.073 g/mol |
|---|
| CAS Registry Number | 86-22-6 |
|---|
| IUPAC Name | [3-(4-bromophenyl)-3-(pyridin-2-yl)propyl]dimethylamine |
|---|
| Traditional Name | brompheniramine |
|---|
| SMILES | CN(C)CCC(C1=CC=C(Br)C=C1)C1=CC=CC=N1 |
|---|
| InChI Identifier | InChI=1S/C16H19BrN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 |
|---|
| InChI Key | ZDIGNSYAACHWNL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pheniramines. Pheniramines are compounds containing a pheniramine moiety, which is structurally characterized by the presence of a 2-benzylpyridine linked to an dimethyl(propyl)amine to form a dimethyl[3-phenyl-3-(pyridin-2-yl)propyl]amine skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pheniramines |
|---|
| Direct Parent | Pheniramines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pheniramine
- Bromobenzene
- Halobenzene
- Aralkylamine
- Aryl bromide
- Benzenoid
- Monocyclic benzene moiety
- Aryl halide
- Heteroaromatic compound
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Organobromide
- Organohalogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | < 25 °C | | Boiling Point | 149.5 °C at 5.00E-01 mm Hg | | Solubility | Freely soluble (maleate salt) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-9081000000-bc4d350922643027fea6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-014i-4954000000-f0a4889ab8cd0d5dcacc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-00di-0090000000-02a74d4d4927d8e443aa | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-014i-0920000000-ad3d20cb0f1b7362fbc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0029000000-fa060b2610f05fd6963a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01b9-0197000000-90a5b62f5a9e47f95802 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fef-3690000000-914dbc7e9fc6b1ce196a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-1bc9f3a3603646c31504 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1019000000-14a32020bd62ee6fb3b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fi4-7591000000-cd391d2ec4970c9e8b8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-615031c9863c28108e0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-6369000000-b40c997a38a0f9eb2fdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-9480000000-6c5bc7bd482fbd292a0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0094000000-c8e61a4d45703ee2c906 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-363f1320fc00a31daef8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-1290000000-3b9b35511af51a9e3bb5 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0002-5490000000-9c3b804de0b552198b85 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Antihistamines are well absorbed from the gastrointestinal tract after oral administration. |
|---|
| Mechanism of Toxicity | Brompheniramine works by acting as an antagonist of the H1 histamine receptors. It also functions as a moderately effective anticholingeric agent, likely an antimuscarinic agent similar to other common antihistamines such as diphenhydramine. Its effects on the cholinergic system may include side-effects such as drowsiness, sedation, dry mouth, dry throat, blurred vision, and increased heart rate. |
|---|
| Metabolism | Hepatic (cytochrome P-450 system), some renal. |
|---|
| Toxicity Values | Oral, Rat: LD50 = 318 mg/kg. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of the symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Signs of overdose include fast or irregular heartbeat, mental or mood changes, tightness in the chest, and unusual tiredness or weakness. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00835 |
|---|
| HMDB ID | HMDB0001941 |
|---|
| FooDB ID | FDB022756 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 1383 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Brompheniramine |
|---|
| Chemspider ID | 6573 |
|---|
| ChEBI ID | 3183 |
|---|
| PubChem Compound ID | 6834 |
|---|
| Kegg Compound ID | C06857 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Sperber, N., Papa, D. and Schwenk, E.; US. Patent 2,567,245; September 11, 1951; assigned
to Schering Corporation.
Sperber, N., Papa, D. and Schwenk, E.; U.S. Patent 2,676,964: April 27,1954; assigned to
Schering Corporation. |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|