| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:47 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003510 |
|---|
| Identification |
|---|
| Common Name | Zoxamide |
|---|
| Class | Small Molecule |
|---|
| Description | Zoxamine is a fungicide used for the control of various fungal inflections including blight in potatoes and tomatoes. It has a rainfast preventative action with residual properties and acts by inhibiting nuclear division. |
|---|
| Contaminant Sources | - My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Fungicide
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
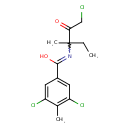
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C14H16Cl3NO2 |
|---|
| Average Molecular Mass | 336.641 g/mol |
|---|
| Monoisotopic Mass | 335.025 g/mol |
|---|
| CAS Registry Number | 156052-68-5 |
|---|
| IUPAC Name | 3,5-dichloro-N-(1-chloro-3-methyl-2-oxopentan-3-yl)-4-methylbenzene-1-carboximidic acid |
|---|
| Traditional Name | 3,5-dichloro-N-(1-chloro-3-methyl-2-oxopentan-3-yl)-4-methylbenzenecarboximidic acid |
|---|
| SMILES | CCC(C)(N=C(O)C1=CC(Cl)=C(C)C(Cl)=C1)C(=O)CCl |
|---|
| InChI Identifier | InChI=1S/C14H16Cl3NO2/c1-4-14(3,12(19)7-15)18-13(20)9-5-10(16)8(2)11(17)6-9/h5-6H,4,7H2,1-3H3,(H,18,20) |
|---|
| InChI Key | SOUGWDPPRBKJEX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dichlorobenzenes. Dichlorobenzenes are compounds containing a benzene with exactly two chlorine atoms attached to it. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Halobenzenes |
|---|
| Direct Parent | Dichlorobenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3-dichlorobenzene
- Toluene
- Aryl chloride
- Aryl halide
- Alpha-haloketone
- Alpha-chloroketone
- Ketone
- Carboximidic acid
- Carboximidic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Alkyl chloride
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Alkyl halide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0229000000-aeaf02faed5a7f957587 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0946000000-d8c6b11940e6700104f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01w0-5930000000-adf1a3e83418ccb730b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0129000000-66c75d1fb00cce7a13ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053s-1495000000-c02eaad9d2632edd79dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zml-9850000000-29b820652a4d4bd580ea | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | The toxicity of zoxamide includes the increase of the weights of liver and thyroid, liver histopathological change and increases in alkaline phosphatase. Zoxamide is classified as “not likely to be carcinogenic to humans” |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 82853 |
|---|
| PubChem Compound ID | 122087 |
|---|
| Kegg Compound ID | C18903 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|