| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:46 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003509 |
|---|
| Identification |
|---|
| Common Name | Triticonazole |
|---|
| Class | Small Molecule |
|---|
| Description | Triticonazole is a seed treatment fungicide for the control of common bunt, loose smut and covered smut on barley, oats and wheat by inhibiting sterol demethylation. |
|---|
| Contaminant Sources | - My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Fungicide
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
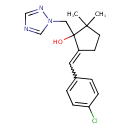
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1Rs)-(e)-5-((4-Chlorophenyl)methylene)-2,2-dimethyl-1-(1H-1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol | MeSH |
|
|---|
| Chemical Formula | C17H20ClN3O |
|---|
| Average Molecular Mass | 317.813 g/mol |
|---|
| Monoisotopic Mass | 317.129 g/mol |
|---|
| CAS Registry Number | 131983-72-7 |
|---|
| IUPAC Name | 5-[(4-chlorophenyl)methylidene]-2,2-dimethyl-1-[(1H-1,2,4-triazol-1-yl)methyl]cyclopentan-1-ol |
|---|
| Traditional Name | 5-[(4-chlorophenyl)methylidene]-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol |
|---|
| SMILES | CC1(C)CCC(=CC2=CC=C(Cl)C=C2)C1(O)CN1C=NC=N1 |
|---|
| InChI Identifier | InChI=1S/C17H20ClN3O/c1-16(2)8-7-14(9-13-3-5-15(18)6-4-13)17(16,22)10-21-12-19-11-20-21/h3-6,9,11-12,22H,7-8,10H2,1-2H3 |
|---|
| InChI Key | PPDBOQMNKNNODG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chlorobenzenes. Chlorobenzenes are compounds containing one or more chlorine atoms attached to a benzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Halobenzenes |
|---|
| Direct Parent | Chlorobenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chlorobenzene
- Aryl chloride
- Aryl halide
- Cyclopentanol
- Azole
- Heteroaromatic compound
- Cyclic alcohol
- 1,2,4-triazole
- Tertiary alcohol
- Azacycle
- Organoheterocyclic compound
- Organochloride
- Organohalogen compound
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1019000000-cc045d670235fec851e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0090000000-26707df997535ec57bcd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9300000000-1cbb9ed860f0d7f42ef8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9002000000-370eaeca40ca8ec94edf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9012000000-bce3b31423022663e784 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-0b9df053e0d2e8f6d514 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Oral (rat) LD50: >2000 mg/kg
Dermal (rat) LD50: >2000 mg/kg
Inhalation (rat) LC50: >1.4 mg/l/4h |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Triticonazole may cause irritation of eyes, skin, respiratory tract and lung injury. It can also cause burns of the esophagus or gastrointestinal tract. In animals (rat, mouse, rabbit and dog), some subchronic and chronic feeding studies show that triticonazole caused adrenal and liver toxicity. It caused some developmental, reproductive toxicity and maternal toxicity in rats and rabbits. Triticonazole was neither genotoxic nor carcinogenic and the U.S. Environmental Protection Agency (U.S. EPA) classified triticonazole as “not likely to be carcinogenic to humans.” |
|---|
| Symptoms | Symptoms include headache, dizziness, weakness, and nausea. |
|---|
| Treatment | For oral exposure, the gastric lavage can be used to remove some of the ingested triticonazole in conscious patients who still have airway protective reflexes and not intubated. Be aware of the potential complications of bleeding or perforation of esophageal or gastrointestinal tract. Activated charcoal can also be used to bind triticonazole and decrease its absorption. Drinking water or water may be of benefit too as soon as ingestion for immediate dilution. (1) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0259283 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 26461628 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|