| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:46 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003507 |
|---|
| Identification |
|---|
| Common Name | Triflumizole |
|---|
| Class | Small Molecule |
|---|
| Description | Triflumizole is a conazole fungicide used to control powdery mildew, scab and other diseases on top fruit, grapes and other crops. Its mode of action is systemic with protective and curative action. It is an inhibitor of chitin biosynthesis. |
|---|
| Contaminant Sources | - My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Ether
- Fungicide
- Organic Compound
- Organochloride
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
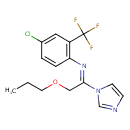
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1E)-N-[4-Chloro-2-(trifluoromethyl)phenyl]-1-(1H-imidazol-1-yl)-2-propoxyethan-1-imine | ChEBI | | (e)-4-Chloro-alpha,alpha,alpha-trifluoro-N-(1-imidazol-1-yl-2-propoxyethylidene)-O-toluidine | ChEBI | | 1-[(1E)-1-[[4-Chloro-2-(trifluoromethyl)phenyl]imino]-2-propoxyethyl]-1H-imidazole | ChEBI | | [N(e)]-4-Chloro-N-[1-(1H-imidazol-1-yl)-2-propoxyethylidene]-2-(trifluoromethyl)benzenamine | ChEBI | | Condor | ChEBI | | NF-114 | ChEBI | | Procure | ChEBI | | Terraguard | ChEBI | | Triflumizol | ChEBI | | Trifumine | ChEBI | | (e)-4-Chloro-a,a,a-trifluoro-N-(1-imidazol-1-yl-2-propoxyethylidene)-O-toluidine | Generator | | (e)-4-Chloro-α,α,α-trifluoro-N-(1-imidazol-1-yl-2-propoxyethylidene)-O-toluidine | Generator |
|
|---|
| Chemical Formula | C15H15ClF3N3O |
|---|
| Average Molecular Mass | 345.747 g/mol |
|---|
| Monoisotopic Mass | 345.086 g/mol |
|---|
| CAS Registry Number | 68694-11-1 and 99387-89-0 |
|---|
| IUPAC Name | (1E)-N-[4-chloro-2-(trifluoromethyl)phenyl]-1-(1H-imidazol-1-yl)-2-propoxyethan-1-imine |
|---|
| Traditional Name | (1E)-N-[4-chloro-2-(trifluoromethyl)phenyl]-1-(imidazol-1-yl)-2-propoxyethanimine |
|---|
| SMILES | CCCOC\C(=N/C1=C(C=C(Cl)C=C1)C(F)(F)F)N1C=CN=C1 |
|---|
| InChI Identifier | InChI=1S/C15H15ClF3N3O/c1-2-7-23-9-14(22-6-5-20-10-22)21-13-4-3-11(16)8-12(13)15(17,18)19/h3-6,8,10H,2,7,9H2,1H3/b21-14+ |
|---|
| InChI Key | HSMVPDGQOIQYSR-KGENOOAVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trifluoromethylbenzenes. These are organofluorine compounds that contain a benzene ring substituted with one or more trifluoromethyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Trifluoromethylbenzenes |
|---|
| Direct Parent | Trifluoromethylbenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trifluoromethylbenzene
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- N-substituted imidazole
- Azole
- Heteroaromatic compound
- Imidazole
- Dialkyl ether
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic oxygen compound
- Organic nitrogen compound
- Alkyl halide
- Alkyl fluoride
- Hydrocarbon derivative
- Organohalogen compound
- Organochloride
- Organofluoride
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Abc transporters | Not Available | map02010 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2009000000-223619f04a5375ff535f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9001000000-83279ba9ad64f257b497 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-cad3b2a3f1872a3d66da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9004000000-15490996bab589c6f985 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9113000000-fbc0f366bc0562a59cae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9441000000-c0009cc1d235a4aebeb2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | In human, a neuromuscular impairment and decreased locomotor activity were observed following a single exposure of 100 mg/kg/day. The liver is the primary target organ of triflumizole in the rat, mouse, and dog. It increased liver weight, hepatocyte fatty vacuolization, hypertrophy, inflammation, fatty degeneration, and caused necrosis. Chronic effects of triflumizole included hepatocyte fatty vacuolization, hepatocyte hypertrophy, focal inflammation, and necrosi,; fatty degeneration, eosinophilic foci of hepatocyte alteration, hepatic nodules, bile duct hyperplasia, and hyaline degeneration/fibrosis of the bile duct. Liver effects were seen in rat and mouse subchronic and chronic/carcinogenicity studies. The fetal effects were also seen in rats. Triflumizole has a reproductive toxicity as well, the gestation length was increased in rats receiving high dose of triflumizole. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 81784 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C18493 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|