| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:46 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003501 |
|---|
| Identification |
|---|
| Common Name | Thidiazuron |
|---|
| Class | Small Molecule |
|---|
| Description | Thidiazuron is an herbicide and defoliant plant growth regulator used particularly in cotton crops. It is absorbed by leaves. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Herbicide
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
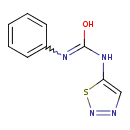
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Phenyl-3-(1,2,3-thiadiazol-5-yl)urea | MeSH | | DROPP | MeSH |
|
|---|
| Chemical Formula | C9H8N4OS |
|---|
| Average Molecular Mass | 220.251 g/mol |
|---|
| Monoisotopic Mass | 220.042 g/mol |
|---|
| CAS Registry Number | 51707-55-2 |
|---|
| IUPAC Name | N'-phenyl-N-(1,2,3-thiadiazol-5-yl)carbamimidic acid |
|---|
| Traditional Name | N'-phenyl-N-(1,2,3-thiadiazol-5-yl)carbamimidic acid |
|---|
| SMILES | OC(NC1=CN=NS1)=NC1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H8N4OS/c14-9(12-8-6-10-13-15-8)11-7-4-2-1-3-5-7/h1-6H,(H2,11,12,14) |
|---|
| InChI Key | HFCYZXMHUIHAQI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-phenylureas. N-phenylureas are compounds containing a N-phenylurea moiety, which is structurally characterized by a phenyl group linked to one nitrogen atom of a urea group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | N-phenylureas |
|---|
| Direct Parent | N-phenylureas |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-phenylurea
- Azole
- Thiadiazole
- Heteroaromatic compound
- Carbonic acid derivative
- Urea
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Tubulin
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Carbon Metabolism | Not Available | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fkc-7960000000-f30d0ea85e4eb18ff693 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-9510000000-5306579d8ff7fab7ae40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ou-9100000000-c78604fd33efcdb8377c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00r6-9830000000-517c3b2d361f98f9e49e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-5900000000-35cf165d6ef594c39abd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-164fb84951f705ce74ab | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Dermal; inhalation. (1) |
|---|
| Mechanism of Toxicity | The US EPA has found no information indicating thidiazuron shares a common mechanism of toxicity with other substances. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | LD50 > 2 g/kg (1). |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Thidiazuron has been registered for use as a pre-harvest cotton defoliant or growth regulator. It removes green leaves and immature fruiting structures, which contribute to cotton staining. (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Relatively few incidents of illness have been reported due to thidiazuron. Since 1992, OPP IDS has only reported 5 incidents as a result of thidiazuron exposure, which all show similar symptoms such as skin rash, nausea, and weakness. However, no medical treatment was required. (1) |
|---|
| Symptoms | Minor skin rash, nausea, and weakness (1). |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Thidiazuron |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 40087 |
|---|
| Kegg Compound ID | C18812 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|