| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:46 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003500 |
|---|
| Identification |
|---|
| Common Name | Thiacloprid |
|---|
| Class | Small Molecule |
|---|
| Description | Thiacloprid is a neonicotinoid insecticide, which is a class of neuro-active insecticides modeled after nicotine. Nicotine was identified and used as an insecticide and rat poison as early as the 1600’s. Its effectiveness as an insecticide spurred a search for insecticidal compounds that have selectively less effect on mammals, which led to the discovery of neonicotinoids. Neonicotinoids, like nicotine, bind to nicotinic acetylcholine receptors of a cell. In mammals, nicotinic acetylcholine receptors are located in cells of both the central and peripheral nervous systems. In insects these receptors are limited to the CNS. While low to moderate activation of these receptors causes nervous stimulation, high levels overstimulate and block the receptors causing paralysis and death. Nicotinic acetylcholine receptors are activated by the neurotransmitter acetylcholine. Acetylcholine is broken down by acetylcholinesterase to terminate signals from these receptors. However, acetylcholinesterase cannot break down neonicotinoids and the binding is irreversible. Because most neonicotinoids bind much more strongly to insect neuron receptors than to mammal neuron receptors, these insecticides are selectively more toxic to insects than mammals. The low mammalian toxicity of neonicotinoids can be explained in large part by their lack of a charged nitrogen atom at physiological pH. The uncharged molecule can penetrate the insect blood–brain barrier, while the mammalian blood–brain barrier filters it. However, Some neonicotinoid breakdown products are toxic to humans, especially if they have become charged. Because of their low toxicity and other favorable features, neonicotinoids are among the most widely used insecticides in the world. Most neonicotinoids are water-soluble and break down slowly in the environment, so they can be taken up by the plant and provide protection from insects as the plant grows. Neonicotinoids are currently used on corn, canola, cotton, sorghum, sugar beets and soybeans. They are also used on the vast majority of fruit and vegetable crops, including apples, cherries, peaches, oranges, berries, leafy greens, tomatoes, and potatoes. The use of neonicotinoids has been linked in a range of studies to adverse ecological effects, including honey-bee colony collapse disorder (CCD) and loss of birds due to a reduction in insect populations. This has led to moratoriums and bans on their use in Europe. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Ether
- Insecticide
- Nitrile
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
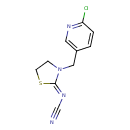
| Chemical Formula | C10H9ClN4S |
|---|
| Average Molecular Mass | 252.723 g/mol |
|---|
| Monoisotopic Mass | 252.024 g/mol |
|---|
| CAS Registry Number | 111988-49-9 |
|---|
| IUPAC Name | {[(2Z)-3-[(6-chloropyridin-3-yl)methyl]-1,3-thiazolidin-2-ylidene]amino}formonitrile |
|---|
| Traditional Name | {[(2Z)-3-[(6-chloropyridin-3-yl)methyl]-1,3-thiazolidin-2-ylidene]amino}formonitrile |
|---|
| SMILES | ClC1=NC=C(CN2CCS\C2=N/C#N)C=C1 |
|---|
| InChI Identifier | InChI=1S/C10H9ClN4S/c11-9-2-1-8(5-13-9)6-15-3-4-16-10(15)14-7-12/h1-2,5H,3-4,6H2/b14-10- |
|---|
| InChI Key | HOKKPVIRMVDYPB-UVTDQMKNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-halopyridines. These are organic compounds containing a pyridine ring substituted at the 2-position by a halogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Halopyridines |
|---|
| Direct Parent | 2-halopyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-halopyridine
- Aryl chloride
- Aryl halide
- Thiazolidine
- Heteroaromatic compound
- Isothiourea
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Azacycle
- Hydrocarbon derivative
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Imine
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cell surface
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 136°C | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-0ab4ce67c5ca41b942df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0690000000-39826f16a704c0d59fe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-4900000000-9adb3574e901a7849274 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-682d029a0271fb73c9b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f76-3950000000-d63990658f3f9d263632 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-bfb39ff24acd4f856c01 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxic cyanide ions or hydrogen cyanide. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). It complexes with the ferric iron atom in this enzyme. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted and the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. Cyanide is also known produce some of its toxic effects by binding to catalase, glutathione peroxidase, methemoglobin, hydroxocobalamin, phosphatase, tyrosinase, ascorbic acid oxidase, xanthine oxidase, succinic dehydrogenase, and Cu/Zn superoxide dismutase. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin. (2) |
|---|
| Metabolism | Organic nitriles are converted into cyanide ions through the action of cytochrome P450 enzymes in the liver. Cyanide is rapidly absorbed and distributed throughout the body. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. Cyanide metabolites are excreted in the urine. (1) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB08620 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Thiacloprid |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 39176 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C18512 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|