| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:44 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003483 |
|---|
| Identification |
|---|
| Common Name | Picloram |
|---|
| Class | Small Molecule |
|---|
| Description | Picloram is a persistent systemic herbicide used for general woody plant control and a broad-leaved weeds on non-crop and utility areas. It also controls a wide range of broad-leaved weeds, but most grasses are resistant. A chlorinated derivative of picolinic acid, picloram is in the pyridine family of herbicides. It is systemically absorbed by roots and leaves and translocated. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- IARC Carcinogens Group 3
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Ester
- Herbicide
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,5,6-Trichloro-4-amino-2-pyridinecarboxylic acid | ChEBI | | 3,5,6-Trichloro-4-aminopicolinic acid | ChEBI | | 4-Amino-3,5,6-trichloro-2-picolinic acid | ChEBI | | 4-Amino-3,5,6-trichloro-2-pyridinecarboxylic acid | ChEBI | | 4-Amino-3,5,6-trichloropicolinic acid | ChEBI | | Access | ChEBI | | Amdon | ChEBI | | Borolin | ChEBI | | Grazon | ChEBI | | K-Pin | ChEBI | | Pathway | ChEBI | | Picloram acid | ChEBI | | Piclorame | ChEBI | | Tordon | ChEBI | | 4-Amino-3,5,6-trichloropyridine-2-carboxylic acid | Kegg | | 3,5,6-Trichloro-4-amino-2-pyridinecarboxylate | Generator | | 3,5,6-Trichloro-4-aminopicolinate | Generator | | 4-Amino-3,5,6-trichloro-2-picolinate | Generator | | 4-Amino-3,5,6-trichloro-2-pyridinecarboxylate | Generator | | 4-Amino-3,5,6-trichloropicolinate | Generator | | 4-Amino-3,5,6-trichloropyridine-2-carboxylate | Generator | | Chloramp | MeSH |
|
|---|
| Chemical Formula | C6H3Cl3N2O2 |
|---|
| Average Molecular Mass | 241.459 g/mol |
|---|
| Monoisotopic Mass | 239.926 g/mol |
|---|
| CAS Registry Number | 1918-02-1 |
|---|
| IUPAC Name | 4-amino-3,5,6-trichloropyridine-2-carboxylic acid |
|---|
| Traditional Name | picloram |
|---|
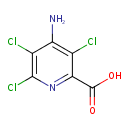
| SMILES | NC1=C(Cl)C(=NC(Cl)=C1Cl)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H3Cl3N2O2/c7-1-3(10)2(8)5(9)11-4(1)6(12)13/h(H2,10,11)(H,12,13) |
|---|
| InChI Key | NQQVFXUMIDALNH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridinecarboxylic acids. Pyridinecarboxylic acids are compounds containing a pyridine ring bearing a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
| Direct Parent | Pyridinecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridine carboxylic acid
- Polyhalopyridine
- Aminopyridine
- 2-halopyridine
- Aryl chloride
- Aryl halide
- Heteroaromatic compound
- Vinylogous halide
- Amino acid or derivatives
- Amino acid
- Carboxylic acid derivative
- Azacycle
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 218.5°C | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0007-3790000000-5cd6ab7784163d17b2ee | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-00di-1900000000-d66381b92085490b74e5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-00ds-9500000000-2b716b50ce950ba0b2ab | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-05fu-0900000000-ffa624570fcf3c060b89 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-05fr-0900000000-8a0464b51afa8f38de47 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0006-0900000000-df7f81b07c2c7ca56034 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0006-0900000000-dfa02aca11bf6b5a48a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0290000000-02d8393ac0289b8d032a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0790000000-816f48ac8e44772246f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f76-0900000000-b15611378d51fb55ce76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0290000000-82cb48a5ba4ec44c28e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000f-0950000000-c86b1f5097d675c9c27e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9700000000-31159cb1112b5e2085f6 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0002-2930000000-8ed140e74455121b4e9d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (1) |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0256531 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Picloram |
|---|
| Chemspider ID | 15170 |
|---|
| ChEBI ID | 34922 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C14310 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|