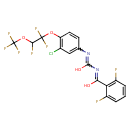| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:44 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003481 |
|---|
| Identification |
|---|
| Common Name | Novaluron |
|---|
| Class | Small Molecule |
|---|
| Description | Novaluron is a chemical with pesticide properties, belonging to the class of new insect growth regulators used to control a range of pests including Lepidoptera, Coleoptera, and Diptera. It is a chitin synthesis inhibitor, with stomach action and some contact activity. In the United States, the compound has been used on food crops, including apples, potatoes, brassicas, ornamentals and cotton. The US Environmental Protection Agency and the Canadian Pest Management Regulatory Agency consider novaluron to pose low risk to the environment and non-target organisms, and value it an important option for integrated pest management that should decrease reliance on organophosphorus, carbamate and pyrethroid insecticides. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Ether
- Food Toxin
- Insecticide
- Organic Compound
- Organochloride
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-[({3-chloro-4-[1,1,2-trifluoro-2-(trifluoromethoxy)ethoxy]phenyl}amino)carbonyl]-2,6-difluorobenzamide | ChEBI | | Rimon | ChEBI | | 1-(3-chloro-4-(1,1,2-trifluoro-2-Trifluoromethoxyethoxy)phenyl)-3-(2,6-difluorobenzoyl)urea | MeSH | | Rimon ec-10 | MeSH |
|
|---|
| Chemical Formula | C17H9ClF8N2O4 |
|---|
| Average Molecular Mass | 492.705 g/mol |
|---|
| Monoisotopic Mass | 492.012 g/mol |
|---|
| CAS Registry Number | 116714-46-6 |
|---|
| IUPAC Name | [({3-chloro-4-[1,1,2-trifluoro-2-(trifluoromethoxy)ethoxy]phenyl}-C-hydroxycarbonimidoyl)imino](2,6-difluorophenyl)methanol |
|---|
| Traditional Name | [({3-chloro-4-[1,1,2-trifluoro-2-(trifluoromethoxy)ethoxy]phenyl}-C-hydroxycarbonimidoyl)imino](2,6-difluorophenyl)methanol |
|---|
| SMILES | OC(N=C(O)C1=C(F)C=CC=C1F)=NC1=CC(Cl)=C(OC(F)(F)C(F)OC(F)(F)F)C=C1 |
|---|
| InChI Identifier | InChI=1S/C17H9ClF8N2O4/c18-8-6-7(4-5-11(8)31-16(22,23)14(21)32-17(24,25)26)27-15(30)28-13(29)12-9(19)2-1-3-10(12)20/h1-6,14H,(H2,27,28,29,30) |
|---|
| InChI Key | NJPPVKZQTLUDBO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenol ethers. These are aromatic compounds containing an ether group substituted with a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenol ethers |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Phenol ethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenoxy compound
- Phenol ether
- Chlorobenzene
- Fluorobenzene
- Halobenzene
- Aryl chloride
- Aryl fluoride
- Aryl halide
- Monocyclic benzene moiety
- Trihalomethane
- Carboximidic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Alkyl fluoride
- Organochloride
- Organohalogen compound
- Organofluoride
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Halomethane
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Alkyl halide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , positive | splash10-0a4l-0900000000-7b5b0dade2c43d3fba16 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , negative | splash10-053r-9405200000-6a4110004c11791b5ccc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , negative | splash10-05gi-5406900000-de2fad8c8161466509f8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , negative | splash10-0ab9-0209700000-f0bbdc0946c8a8f66828 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , negative | splash10-05fr-0204900000-bd3521813fc9e7158d37 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - NA , negative | splash10-0uk9-0138900000-86230266df1e3feda377 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0704900000-19a03abb77c78dda48ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-0902100000-b1bcf3b85c3c70ee2099 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-0900000000-a5690ffa4fae01781edd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6u-0409400000-454ec0eb088610821068 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a70-0916200000-92e1ba3234d1fac36a95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1910000000-15a693e28777b8dfa0be | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Novaluron |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 39385 |
|---|
| PubChem Compound ID | 93541 |
|---|
| Kegg Compound ID | C18875 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|