| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:43 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003474 |
|---|
| Identification |
|---|
| Common Name | Hexaconazole |
|---|
| Class | Small Molecule |
|---|
| Description | Hexaconazole is a systemic conazole (imidazole) fungicide used for the control of many seed-borne and soil-borne diseases and fungi particularly Ascomycetes and Basidiomycetes. It is commonly used in the control of apple, coffee and peanut diseases. It has a broad spectrum of action, being systemic with protective and curative action. It is known to disrupt membrane function. |
|---|
| Contaminant Sources | - My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Fungicide
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
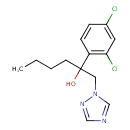
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C14H17Cl2N3O |
|---|
| Average Molecular Mass | 314.210 g/mol |
|---|
| Monoisotopic Mass | 313.075 g/mol |
|---|
| CAS Registry Number | 79983-71-4 |
|---|
| IUPAC Name | 2-(2,4-dichlorophenyl)-1-(1H-1,2,4-triazol-1-yl)hexan-2-ol |
|---|
| Traditional Name | hexaconazole |
|---|
| SMILES | CCCCC(O)(CN1C=NC=N1)C1=C(Cl)C=C(Cl)C=C1 |
|---|
| InChI Identifier | InChI=1S/C14H17Cl2N3O/c1-2-3-6-14(20,8-19-10-17-9-18-19)12-5-4-11(15)7-13(12)16/h4-5,7,9-10,20H,2-3,6,8H2,1H3 |
|---|
| InChI Key | STMIIPIFODONDC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dichlorobenzenes. Dichlorobenzenes are compounds containing a benzene with exactly two chlorine atoms attached to it. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Halobenzenes |
|---|
| Direct Parent | Dichlorobenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3-dichlorobenzene
- Aryl chloride
- Aryl halide
- Azole
- Tertiary alcohol
- 1,2,4-triazole
- Heteroaromatic compound
- Organoheterocyclic compound
- Azacycle
- Aromatic alcohol
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Extracellular
- Membrane
- Plasma Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1139000000-85655c8ede9b6f33b510 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1090000000-c88e37855ef286924d19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-9000000000-fe32b63be3e0493c3194 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9022000000-91330ab3c9f4bae9c9ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9021000000-4f00fe56f542dd0c016f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-5aae658e2bcd0ef74ae6 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-001i-9640000000-5dc43bf46269dbe787d3 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Hexaconazole |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 83711 |
|---|
| PubChem Compound ID | 66461 |
|---|
| Kegg Compound ID | C18466 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|