| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:42 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003459 |
|---|
| Identification |
|---|
| Common Name | Dithiopyr |
|---|
| Class | Small Molecule |
|---|
| Description | Dithiopyr is a chemical used as a pre-emergent herbicide used to prevent crabgrass seeds from sprouting in the spring. Dithiopyr may alter microtubule polymerization and stability by interacting with microtubule associated proteins and/or microtubule organizing centers rather than interaction directly with tubulin. |
|---|
| Contaminant Sources | - My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Ester
- Ether
- Herbicide
- Household Toxin
- Organic Compound
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
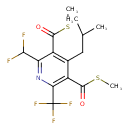
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H16F5NO2S2 |
|---|
| Average Molecular Mass | 401.415 g/mol |
|---|
| Monoisotopic Mass | 401.054 g/mol |
|---|
| CAS Registry Number | 97886-45-8 |
|---|
| IUPAC Name | [6-(difluoromethyl)-4-(2-methylpropyl)-5-[(methylsulfanyl)carbonyl]-2-(trifluoromethyl)pyridin-3-yl](methylsulfanyl)methanone |
|---|
| Traditional Name | dithiopyr |
|---|
| SMILES | CSC(=O)C1=C(CC(C)C)C(C(=O)SC)=C(N=C1C(F)F)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C15H16F5NO2S2/c1-6(2)5-7-8(13(22)24-3)10(12(16)17)21-11(15(18,19)20)9(7)14(23)25-4/h6,12H,5H2,1-4H3 |
|---|
| InChI Key | YUBJPYNSGLJZPQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridinecarboxylic acids and derivatives. Pyridinecarboxylic acids and derivatives are compounds containing a pyridine ring bearing a carboxylic acid group or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
| Direct Parent | Pyridinecarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridine carboxylic acid or derivatives
- Heteroaromatic compound
- Thiocarboxylic acid ester
- Carbothioic s-ester
- Carboxylic acid derivative
- Thiocarboxylic acid or derivatives
- Sulfenyl compound
- Azacycle
- Organic oxygen compound
- Alkyl halide
- Organic nitrogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Organopnictogen compound
- Alkyl fluoride
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009700000-408df4aecad29ef0c335 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-2009100000-e918f720fb4ca734bae9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-4049000000-3349a9b3bcaadd99aa26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udj-6009500000-437c36e80bd37469ded6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uea-3009100000-4a1763273cfdf3ba65ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9006000000-53723b12b9ac826e30f6 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dithiopyr |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 91757 |
|---|
| Kegg Compound ID | C18826 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|