| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 05:02:45 UTC |
|---|
| Update Date | 2016-11-09 01:09:02 UTC |
|---|
| Accession Number | CHEM003031 |
|---|
| Identification |
|---|
| Common Name | Gelsemine |
|---|
| Class | Small Molecule |
|---|
| Description | Gelsemine is a toxic alkaloid found in the plant Gelsemium sempervirens. It has convulsant effects with a similar mechanism of action to strychnine. Due to its complex structure, gelsemine has been the target for a number of total synthesis projects. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Ether
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
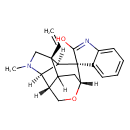
| Chemical Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | C20H22N2O2 |
|---|
| Average Molecular Mass | 322.401 g/mol |
|---|
| Monoisotopic Mass | 322.168 g/mol |
|---|
| CAS Registry Number | 509-15-9 |
|---|
| IUPAC Name | (2'S,3S,5'S,6'S,8'R,11'S)-2'-ethenyl-4'-methyl-9'-oxa-4'-azaspiro[indole-3,7'-tetracyclo[6.3.1.0²,⁶.0⁵,¹¹]dodecane]-2-ol |
|---|
| Traditional Name | (2'S,3S,5'S,6'S,8'R,11'S)-2'-ethenyl-4'-methyl-9'-oxa-4'-azaspiro[indole-3,7'-tetracyclo[6.3.1.0²,⁶.0⁵,¹¹]dodecane]-2-ol |
|---|
| SMILES | [H][C@]12CO[C@]3([H])CC1([H])[C@@]1(CN(C)[C@@]2([H])[C@]1([H])[C@@]31C(O)=NC2=CC=CC=C12)C=C |
|---|
| InChI Identifier | InChI=1S/C20H22N2O2/c1-3-19-10-22(2)16-11-9-24-15(8-13(11)19)20(17(16)19)12-6-4-5-7-14(12)21-18(20)23/h3-7,11,13,15-17H,1,8-10H2,2H3,(H,21,23)/t11-,13?,15+,16+,17-,19-,20-/m0/s1 |
|---|
| InChI Key | NFYYATWFXNPTRM-ICFOCEFXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gelsemium alkaloids. These are alkaloids with a structure that is based on the tetracyclic gelsemium skeleton. These alkaloids contain an oxindole function and a cage-like, hydroaromatic residue which is believed to arise from an intermediate related to anhydrovobasinediol by formation of a 6,20 bond and rearrangement to an oxindole. The major alkaloids in this group are related to Gelsemine; however, a smaller group, characterized by Gelsedine, lack the 6,20 bond, and have also lost C-21. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Gelsemium alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Gelsemium alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gelsemium skeleton
- Indole or derivatives
- Dihydroindole
- Isoindoline
- Isoindole or derivatives
- Oxepane
- Aralkylamine
- Oxane
- Piperidine
- N-alkylpyrrolidine
- Benzenoid
- Pyrrolidine
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Azacycle
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0019000000-2d59eb57ec3278195ff1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-0259000000-5bdc5c9f0ec742533434 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-8920000000-ff7118dd6e7438a2adf6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-3a4e2cd4e51f80284734 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-3019000000-f600237c1ff6849cd301 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9230000000-c3c63118a4dbe4571005 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Gelsemine has convulsant effects with a similar mechanism of action to strychnine. Strychnine is a neurotoxin which acts as an antagonist of glycine and acetylcholine receptors. It primarily affects the motor nerves in the spinal cord which control muscle contraction. An impulse is triggered at one end of a nerve by the binding of neurotransmitters to the receptors. In the presence of a neuroinhibitor, such as glycine, a greater quantity of excitatory neurotransmitters must bind to receptors before there will be an action potential generated. Glycine acts primarily as an agonist of the glycine receptor, which is a ligand-gated chloride channel in neurons located in the spinal cord and in the brain. This chloride channel will allow the negatively charged chloride ions into the neuron, causing a hyperpolarization which pushes the membrane potential further from threshold. Strychnine is an antagonist of glycine, which means it binds to the same receptor, preventing the inhibitory effects of glycine on the postsynaptic neuron. Therefore, action potentials are triggered with lower levels of excitatory neurotransmitters. When the inhibitory signals are prevented, the motor neurons do will be more easliy activated and the victim will have spastic muscle contractions. Structure of strychnine in complex with ACh binding protein (AChBP). Strychnine is also an antagonist for acetylcholine receptors, which is known to be homologous to the glycine receptor. (Wikipedia) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Gelsemine is a toxic alkaloid found in the plant Gelsemium sempervirens. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001734 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Gelsemine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C09207 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|