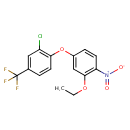| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:53 UTC |
|---|
| Update Date | 2016-11-09 01:08:59 UTC |
|---|
| Accession Number | CHEM002857 |
|---|
| Identification |
|---|
| Common Name | Oxyfluorfen |
|---|
| Class | Small Molecule |
|---|
| Description | Oxyfluorfen is a selective pre and postemergent herbicide used to control certain annual broadleaf and grassy weeds in vegetables, fruit, cotton, es] ornamentals and on non-crop areas. It is a contact herbicide and light is required for it to affect target plants. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Ether
- Herbicide
- Household Toxin
- Organic Compound
- Organochloride
- Organofluoride
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Oxyfluorofen | MeSH |
|
|---|
| Chemical Formula | C15H11ClF3NO4 |
|---|
| Average Molecular Mass | 361.700 g/mol |
|---|
| Monoisotopic Mass | 361.033 g/mol |
|---|
| CAS Registry Number | 42874-03-3 |
|---|
| IUPAC Name | 4-[2-chloro-4-(trifluoromethyl)phenoxy]-2-ethoxy-1-nitrobenzene |
|---|
| Traditional Name | oxyfluorfen |
|---|
| SMILES | CCOC1=C(C=CC(OC2=C(Cl)C=C(C=C2)C(F)(F)F)=C1)[N+]([O-])=O |
|---|
| InChI Identifier | InChI=1S/C15H11ClF3NO4/c1-2-23-14-8-10(4-5-12(14)20(21)22)24-13-6-3-9(7-11(13)16)15(17,18)19/h3-8H,2H2,1H3 |
|---|
| InChI Key | OQMBBFQZGJFLBU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylethers. These are aromatic compounds containing two benzene rings linked to each other through an ether group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylethers |
|---|
| Direct Parent | Diphenylethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylether
- Nitrophenyl ether
- Diaryl ether
- Trifluoromethylbenzene
- Nitrobenzene
- Phenoxy compound
- Nitroaromatic compound
- Phenol ether
- Alkyl aryl ether
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Organic nitro compound
- C-nitro compound
- Ether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Organohalogen compound
- Alkyl halide
- Alkyl fluoride
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organochloride
- Organofluoride
- Organonitrogen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003r-1769000000-df977b3ebe5088cb8b0b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-01ql-0940000000-b695f029454204c9d39f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-000i-0690000000-1e8071fd6881b97d3362 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0f79-0190000000-01510ce51e7f0a0a6d96 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0fri-0093000000-788cecb0eb84ed3961b8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0009000000-488df719a013b26bbc89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-ab5b7890aae942f41d8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0009000000-ebd8ec6f39e9a5bc8e98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-005i-1906000000-837b5a3e9656688912ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-42248a28f4761c28acb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-79e99a0e9a4180518bfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-7915000000-6cc60a27948c0897f634 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0w29-5198000000-e217309804cf3e3d7968 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0256013 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35974 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C18881 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|