| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:53 UTC |
|---|
| Update Date | 2016-11-09 01:08:59 UTC |
|---|
| Accession Number | CHEM002856 |
|---|
| Identification |
|---|
| Common Name | Oxasulfuron |
|---|
| Class | Small Molecule |
|---|
| Description | Oxasulfuron is a short residual post-emergence herbicide intended for treating crops such as soybeans. It is taken up by the roots and shoots and translocated to the meristematic tissue. Treated weeds die 1-3 weeks after application. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Ester
- Ether
- Herbicide
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
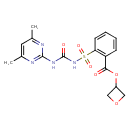
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Oxasulphuron | Generator |
|
|---|
| Chemical Formula | C17H18N4O6S |
|---|
| Average Molecular Mass | 406.413 g/mol |
|---|
| Monoisotopic Mass | 406.095 g/mol |
|---|
| CAS Registry Number | 144651-06-9 |
|---|
| IUPAC Name | oxetan-3-yl 2-({[(4,6-dimethylpyrimidin-2-yl)carbamoyl]amino}sulfonyl)benzoate |
|---|
| Traditional Name | oxasulfuron |
|---|
| SMILES | CC1=CC(C)=NC(NC(=O)NS(=O)(=O)C2=C(C=CC=C2)C(=O)OC2COC2)=N1 |
|---|
| InChI Identifier | InChI=1S/C17H18N4O6S/c1-10-7-11(2)19-16(18-10)20-17(23)21-28(24,25)14-6-4-3-5-13(14)15(22)27-12-8-26-9-12/h3-7,12H,8-9H2,1-2H3,(H2,18,19,20,21,23) |
|---|
| InChI Key | IOXAXYHXMLCCJJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidinyl-2-sulfonylureas. These are aromatic heterocyclic compounds containing a pyrimidine ring which is substituted with a sulfonylurea at the ring 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Sulfonylureas |
|---|
| Direct Parent | Pyrimidinyl-2-sulfonylureas |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidinyl-2-sulfonylurea
- Benzenesulfonamide
- Benzoate ester
- Benzoic acid or derivatives
- Benzenesulfonyl group
- Benzoyl
- Monocyclic benzene moiety
- Pyrimidine
- Benzenoid
- Heteroaromatic compound
- Organic sulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Organosulfonic acid or derivatives
- Carboxylic acid ester
- Carbonic acid derivative
- Oxetane
- Monocarboxylic acid or derivatives
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organosulfur compound
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-7393800000-ad44cc0de7a6e35a8427 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-9670000000-8f134635642f3503d81d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pdi-7901000000-e6f5d0992868d94e6072 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ab9-6935600000-15084f1d7bffa0caf157 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3592100000-faa91ea2c10de384794f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-2972000000-d5264bf70b5865375c0d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 86443 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|