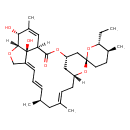| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:53 UTC |
|---|
| Update Date | 2016-11-09 01:08:59 UTC |
|---|
| Accession Number | CHEM002848 |
|---|
| Identification |
|---|
| Common Name | Milbemectin (mixture) |
|---|
| Class | Small Molecule |
|---|
| Description | Milbemectin is a mixture of milbemycin A3 and milbemycin A4 in a ratio of 3:7. It is an active acaricide produced from fermentation products of Streptomyces subspecies. It is highly active on various phytophagous mites that live on tea plants, pome fruits, stone fruits, citrus, watermelon, strawberry and egg plants. It is also an insecticide that is active against aphids, cutworms, leafrollers and thrips. It appears to stimulate the release of gamma-aminobutyric acid (GABA) which affects the chloride channels of the central nervous system. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Ester
- Ether
- Insecticide
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Hydroxymilbemycin beta7 | MeSH | | Milbemycin alpha7 | MeSH | | Milbemycin alpha2 | MeSH | | Milbemycin alpha9 | MeSH | | Moxidectin | MeSH | | Milbemycin alpha13 | MeSH | | Milbemycin beta3 | MeSH | | Milbemycin b | MeSH | | Milbemycin beta12 | MeSH | | Milbemycin D | MeSH | | Milbemycin alpha3 | MeSH | | Milbemycin alpha6 | MeSH | | Milbemycins | MeSH | | Milbemectin | MeSH | | Milbemycin | MeSH | | Milbemycin alpha8 | MeSH | | Milbemycin beta2 | MeSH | | Milbemycin alpha11 | MeSH | | Milbemycin alpha1 | MeSH | | Milbemycin alpha5 | MeSH | | Milbemycin beta1 | MeSH | | Milbemycin a3 | MeSH | | Milbemycin alpha4 | MeSH | | Milbemycin alpha10 | MeSH | | Cydectin | MeSH | | Milbemycin alpha14 | MeSH | | Milbemycin alpha15 | MeSH | | Milbemycin a4 | MeSH |
|
|---|
| Chemical Formula | C32H46O7 |
|---|
| Average Molecular Mass | 542.703 g/mol |
|---|
| Monoisotopic Mass | 542.324 g/mol |
|---|
| CAS Registry Number | 51596-11-3 |
|---|
| IUPAC Name | (1'R,2R,4'S,5S,6R,8'R,10'E,13'R,14'E,20'R,21'R,24'S)-6-ethyl-21',24'-dihydroxy-5,11',13',22'-tetramethyl-3',7',19'-trioxaspiro[oxane-2,6'-tetracyclo[15.6.1.1⁴,⁸.0²⁰,²⁴]pentacosane]-10',14',16',22'-tetraen-2'-one |
|---|
| Traditional Name | (1'R,2R,4'S,5S,6R,8'R,10'E,13'R,14'E,20'R,21'R,24'S)-6-ethyl-21',24'-dihydroxy-5,11',13',22'-tetramethyl-3',7',19'-trioxaspiro[oxane-2,6'-tetracyclo[15.6.1.1⁴,⁸.0²⁰,²⁴]pentacosane]-10',14',16',22'-tetraen-2'-one |
|---|
| SMILES | [H][C@@]12OC\C3=C/C=C/[C@H](C)C\C(C)=C\C[C@]4([H])C[C@@H](C[C@]5(CC[C@H](C)[C@@H](CC)O5)O4)OC(=O)[C@]([H])(C=C(C)[C@H]1O)[C@@]23O |
|---|
| InChI Identifier | InChI=1S/C32H46O7/c1-6-27-21(4)12-13-31(39-27)17-25-16-24(38-31)11-10-20(3)14-19(2)8-7-9-23-18-36-29-28(33)22(5)15-26(30(34)37-25)32(23,29)35/h7-10,15,19,21,24-29,33,35H,6,11-14,16-18H2,1-5H3/b8-7+,20-10+,23-9+/t19-,21-,24+,25-,26-,27+,28+,29+,31+,32+/m0/s1 |
|---|
| InChI Key | VOZIAWLUULBIPN-LRBNAKOISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as milbemycins. These are a group of macrolides with a structure containing a 16-membered lactone ring fused to a 1,7-dioxaspiroundecane ring system and to either a benzofuran (or hydrogenated derivative thereof). In some cases (e.g. Milbemycin E), the tetrahydrofuranyl ring is missing. Milbemycins can be o-glycosylated at C13 to form Avermectins. Milbemycins are produced by Streptomyces species. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolides and analogues |
|---|
| Sub Class | Milbemycins |
|---|
| Direct Parent | Milbemycins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Milbemycin
- Ketal
- Oxane
- Tetrahydrofuran
- Tertiary alcohol
- Carboxylic acid ester
- Lactone
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-3100290000-c0537502ba618e2d28d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9222020000-1bfbe716b5d7338aefb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003g-9121200000-0db3aa9765bd3fe72eff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2003290000-d7f9b77aec3f53771d73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bc-0346950000-f6325e67be278daaa209 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kka-4019310000-0a312399e60dfbdb402b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 39229 |
|---|
| PubChem Compound ID | 9959038 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|