| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:53 UTC |
|---|
| Update Date | 2016-11-09 01:08:59 UTC |
|---|
| Accession Number | CHEM002846 |
|---|
| Identification |
|---|
| Common Name | MGK |
|---|
| Class | Small Molecule |
|---|
| Description | N-Octyl bicycloheptene dicarboximide (MGK 264) is an ingredient in some common pesticides. It has no intrinsic pesticidal activity itself, but rather is a synergist enhancing the potency of pyrethroid ingredients. It is used in a variety of household and veterinary products |
|---|
| Contaminant Sources | - EPA Endocrine Screening
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Household Toxin
- Organic Compound
- Synthetic Compound
|
|---|
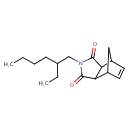
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Octylbicycloheptene dicarboximide | Kegg | | MGK-264 | HMDB | | MGK 264 | HMDB | | N-(2-Ethylhexyl)-5-norbornene-2,3-dicarboxamide | HMDB | | Ethylhexylbicycloheptene dicarboximide | HMDB |
|
|---|
| Chemical Formula | C17H25NO2 |
|---|
| Average Molecular Mass | 275.386 g/mol |
|---|
| Monoisotopic Mass | 275.189 g/mol |
|---|
| CAS Registry Number | 113-48-4 |
|---|
| IUPAC Name | 4-(2-ethylhexyl)-4-azatricyclo[5.2.1.0²,⁶]dec-8-ene-3,5-dione |
|---|
| Traditional Name | 4-(2-ethylhexyl)-4-azatricyclo[5.2.1.0²,⁶]dec-8-ene-3,5-dione |
|---|
| SMILES | CCCCC(CC)CN1C(=O)C2C3CC(C=C3)C2C1=O |
|---|
| InChI Identifier | InChI=1S/C17H25NO2/c1-3-5-6-11(4-2)10-18-16(19)14-12-7-8-13(9-12)15(14)17(18)20/h7-8,11-15H,3-6,9-10H2,1-2H3 |
|---|
| InChI Key | WLLGXSLBOPFWQV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoindolones. These are aromatic polycyclic compounds that an isoindole bearing a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoindoles and derivatives |
|---|
| Sub Class | Isoindolines |
|---|
| Direct Parent | Isoindolones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoindolone
- Carboxylic acid imide, n-substituted
- Pyrrolidone
- 2-pyrrolidone
- N-alkylpyrrolidine
- Carboxylic acid imide
- Dicarboximide
- Pyrrolidine
- Lactam
- Carboxylic acid derivative
- Azacycle
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0694-9440000000-40454705bcde83d5fda4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1490000000-95e5dd3401ddf362ec17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01tc-6490000000-6c6aae6faad89269ac6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-21da35f03c4473badc52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0229-0690000000-9b7810dfc603ba0831a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1930000000-3c0254e457a0bf1672a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dv-9700000000-8aa1972a3ea8b0afb560 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-03xr-9510000000-d0247447731c32abc9a3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | It is used in a variety of household and veterinary products. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0256988 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Machine Gun Kelly (musician) |
|---|
| Chemspider ID | 7934 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 8227 |
|---|
| Kegg Compound ID | C18795 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|