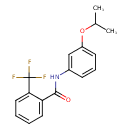| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:52 UTC |
|---|
| Update Date | 2016-11-09 01:08:58 UTC |
|---|
| Accession Number | CHEM002813 |
|---|
| Identification |
|---|
| Common Name | Flutolanil |
|---|
| Class | Small Molecule |
|---|
| Description | Flutolanil is a systemic fungicide. It is an inhibitor of an enzyme complex that is required for the respiration, and thus inhibits the synthesis of glutamate and aspartate. Flutolanil is used as a dusting powder for disinfection of seed potatoes before or during planting, against black scurf. Flutolanil has low acute toxicity to mammals. It is not genotoxic, carcinogenic or teratogenic. |
|---|
| Contaminant Sources | - EPA Endocrine Screening
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Ester
- Ether
- Fungicide
- Organic Compound
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Isopropoxy-2-trifloromethylbenzoic acid anilide | ChEBI | | alpha,alpha,alpha-Trifluoro-3'-isopropoxy-O-toluanilide | ChEBI | | Moncut | ChEBI | | N-(3-(1-Methylethoxy)phenyl)-2-(trifluoromethyl)benzamide | ChEBI | | N-(3-Isopropoxyphenyl)-2-(trifluoromethyl)benzamide | ChEBI | | NNF-136 | ChEBI | | O-Trifluoromethyl-m'-isopropoxybenzoic anilide | ChEBI | | 3'-Isopropoxy-2-trifloromethylbenzoate anilide | Generator | | a,a,a-Trifluoro-3'-isopropoxy-O-toluanilide | Generator | | Α,α,α-trifluoro-3'-isopropoxy-O-toluanilide | Generator |
|
|---|
| Chemical Formula | C17H16F3NO2 |
|---|
| Average Molecular Mass | 323.310 g/mol |
|---|
| Monoisotopic Mass | 323.113 g/mol |
|---|
| CAS Registry Number | 66332-96-5 |
|---|
| IUPAC Name | N-[3-(propan-2-yloxy)phenyl]-2-(trifluoromethyl)benzamide |
|---|
| Traditional Name | flutolanil |
|---|
| SMILES | CC(C)OC1=CC=CC(NC(=O)C2=CC=CC=C2C(F)(F)F)=C1 |
|---|
| InChI Identifier | InChI=1S/C17H16F3NO2/c1-11(2)23-13-7-5-6-12(10-13)21-16(22)14-8-3-4-9-15(14)17(18,19)20/h3-11H,1-2H3,(H,21,22) |
|---|
| InChI Key | PTCGDEVVHUXTMP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzanilides. These are aromatic compounds containing an anilide group in which the carboxamide group is substituted with a benzene ring. They have the general structure RNC(=O)R', where R,R'= benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Anilides |
|---|
| Direct Parent | Benzanilides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzanilide
- Trifluoromethylbenzene
- Benzamide
- Benzoic acid or derivatives
- Phenoxy compound
- Benzoyl
- Phenol ether
- Alkyl aryl ether
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Ether
- Organic nitrogen compound
- Organofluoride
- Organohalogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Alkyl halide
- Alkyl fluoride
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0339000000-861dd8a6b97fd2380469 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1492000000-9cfdb354aef2c510963c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f72-4920000000-0aab7187f80b2e3b91c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0109000000-339b8456cc6bfb6009ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0639000000-7fadd9acddac4f8dd8d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001j-1920000000-ebbdd99d53b507137228 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00di-1910000000-700f65851992dc9b3764 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-15890 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 81792 |
|---|
| PubChem Compound ID | 47898 |
|---|
| Kegg Compound ID | C18502 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|