| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:51 UTC |
|---|
| Update Date | 2016-11-09 01:08:58 UTC |
|---|
| Accession Number | CHEM002792 |
|---|
| Identification |
|---|
| Common Name | Emamectin benzoate |
|---|
| Class | Small Molecule |
|---|
| Description | Emamectin benzoate (Proclaim) is an avermectin class insecticide developed for the control of lepidopteron insects. This class of pesticide consists of homologous semi-synthetic macrolides that are derived from the natural fermentation products of Streptomyces bacteria. It kills insects by disrupting neurotransmitters, causing irreversible paralysis. It is more effective when ingested, but it also somewhat effective on contact. When sprayed to foliage, emamectin benzoate penetrates the leaf tissue and forms reservoir within treated leaves, which provides residual activity against pests that ingest the substance when feeding. Emamectin is widely used in the US and Canada as an insecticide because of its chloride channel activation properties. It is approved by the EPA for use in prevention of emerald ash borer in ash trees. Emamectin has also shown promising applications in the eradication of fish lice and in fish farming. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | - Insecticide
- Pesticide
- Synthetic Compound
|
|---|
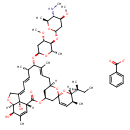
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Emamectin benzoic acid | Generator | | 4''-Deoxy-4''-epi-N-methylaminoavermectin b1 benzoate | MeSH |
|
|---|
| Chemical Formula | C56H81NO15 |
|---|
| Average Molecular Mass | 1008.240 g/mol |
|---|
| Monoisotopic Mass | 1007.561 g/mol |
|---|
| CAS Registry Number | 155569-91-8 |
|---|
| IUPAC Name | (2S,3S,4S,6S)-6-{[(2S,3S,4S,6R)-6-[(1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-butan-2-yl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-5,6-dihydro-3',7',19'-trioxaspiro[pyran-2,6'-tetracyclo[15.6.1.1⁴,⁸.0²⁰,²⁴]pentacosane]-10',14',16',22'-tetraen-2'-oneoxy]-4-methoxy-2-methyloxan-3-yl]oxy}-4-methoxy-N,2-dimethyloxan-3-aminium benzoate |
|---|
| Traditional Name | (2S,3S,4S,6S)-6-{[(2S,3S,4S,6R)-6-[(1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-butan-2-yl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-5,6-dihydro-3',7',19'-trioxaspiro[pyran-2,6'-tetracyclo[15.6.1.1⁴,⁸.0²⁰,²⁴]pentacosane]-10',14',16',22'-tetraen-2'-oneoxy]-4-methoxy-2-methyloxan-3-yl]oxy}-4-methoxy-N,2-dimethyloxan-3-aminium benzoate |
|---|
| SMILES | [O-]C(=O)C1=CC=CC=C1.[H][C@](C)(CC)[C@@]1([H])O[C@@]2(C[C@@H]3C[C@@]([H])(C\C=C(C)\[C@@H](O[C@H]4C[C@H](OC)[C@@H](O[C@H]5C[C@H](OC)[C@@H]([NH2+]C)[C@H](C)O5)[C@H](C)O4)[C@@H](C)\C=C\C=C4/CO[C@]5([H])[C@H](O)C(C)=C[C@@]([H])(C(=O)O3)[C@]45O)O2)C=C[C@@H]1C |
|---|
| InChI Identifier | InChI=1S/C49H75NO13.C7H6O2/c1-12-26(2)44-29(5)18-19-48(63-44)24-35-21-34(62-48)17-16-28(4)43(27(3)14-13-15-33-25-56-46-42(51)30(6)20-36(47(52)59-35)49(33,46)53)60-40-23-38(55-11)45(32(8)58-40)61-39-22-37(54-10)41(50-9)31(7)57-39;8-7(9)6-4-2-1-3-5-6/h13-16,18-20,26-27,29,31-32,34-46,50-51,53H,12,17,21-25H2,1-11H3;1-5H,(H,8,9)/b14-13+,28-16+,33-15+;/t26-,27-,29-,31-,32-,34+,35-,36-,37-,38-,39-,40-,41-,42+,43-,44+,45-,46+,48+,49+;/m0./s1 |
|---|
| InChI Key | GCKZANITAMOIAR-XWVCPFKXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aminoglycosides. These are molecules or a portion of a molecule composed of amino-modified sugars. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Aminoglycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminoglycoside core
- Macrolide
- Glycosyl compound
- O-glycosyl compound
- Benzoic acid or derivatives
- Benzoic acid
- Benzoyl
- Ketal
- Benzenoid
- Pyran
- Oxane
- Monocyclic benzene moiety
- Monosaccharide
- Tetrahydrofuran
- Tertiary alcohol
- Amino acid or derivatives
- Secondary alcohol
- Carboxylic acid ester
- Lactone
- Organoheterocyclic compound
- Secondary amine
- Oxacycle
- Acetal
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Dialkyl ether
- Secondary aliphatic amine
- Ether
- Carbonyl group
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Amine
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-59be3ae3b24c8cbd6bb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-59be3ae3b24c8cbd6bb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-59be3ae3b24c8cbd6bb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-70b44bba6aa0336bce93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-70b44bba6aa0336bce93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-70b44bba6aa0336bce93 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Emamectin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11650986 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|