| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:50 UTC |
|---|
| Update Date | 2016-11-09 01:08:58 UTC |
|---|
| Accession Number | CHEM002783 |
|---|
| Identification |
|---|
| Common Name | Dazomet |
|---|
| Class | Small Molecule |
|---|
| Description | Dazomet is a common soil fumigant that acts as a herbicide, fungicide, and nematicide. Dazomet is used as a soil sterilant on a variety of sites such as golf courses, nurseries, turf sites, and potting soils.[2] Dazomet is used for soil sterilization as an alternative to methyl bromide. Although less effective it is still used to kill pests because of its comparatively lower toxicity. Dazomet is applied to wet soil, which causes dazomet itself to decompose into a gaseous form, which is what actively controls pests. The decomposition of dazomet releases methyl isothiocyanate (MITC) a gas toxic to pests that would prevent or kill plant growth. Dazomet is irritating to the eyes and its degradation product, MITC, is a dermal sensitizer. Dazomet is very toxic to aquatic organisms, and also acutely toxic to mammals. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Carbamate
- Ester
- Ether
- Fungicide
- Lachrymator
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
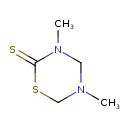
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Thio-3,5-dimethyltetrahydro-1,3,5-thiadiazine | ChEBI | | 3,5-Dimethyl-1,3,5-(2H)-tetrahydrothiadiazine-2-thione | ChEBI | | 3,5-Dimethyl-2-thionotetrahydro-1,3,5-thiadiazine | ChEBI | | 3,5-Dimethyltetrahydro-1,3,5-2H-thiadiazine-2-thione | ChEBI | | 3,5-Dimethyltetrahydro-1,3,5-thiadiazine-2-thione | ChEBI | | 3,5-Dimethyltetrahydro-2H-1,3,5-thiadiazine-2-thione | ChEBI | | Basamid | ChEBI | | Crag 974 | ChEBI | | Dazoberg | ChEBI | | Dimethylformocarbothialdine | ChEBI | | DMTT | ChEBI | | Mylone | ChEBI | | NSC 4737 | ChEBI | | Tetrahydro-2H-3,5-dimethyl-1,3,5-thiadiazine-2-thione | ChEBI | | Tetrahydro-3,5-dimethyl-2H-1,3,5-thiadiazine-2-thione | ChEBI | | Tiazon | ChEBI | | UCC 974 | ChEBI | | Thiazone | MeSH | | Basamide | MeSH |
|
|---|
| Chemical Formula | C5H10N2S2 |
|---|
| Average Molecular Mass | 162.276 g/mol |
|---|
| Monoisotopic Mass | 162.029 g/mol |
|---|
| CAS Registry Number | 533-74-4 |
|---|
| IUPAC Name | 3,5-dimethyl-1,3,5-thiadiazinane-2-thione |
|---|
| Traditional Name | thiazon |
|---|
| SMILES | CN1CSC(=S)N(C)C1 |
|---|
| InChI Identifier | InChI=1S/C5H10N2S2/c1-6-3-7(2)5(8)9-4-6/h3-4H2,1-2H3 |
|---|
| InChI Key | QAYICIQNSGETAS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiadiazinanes. These are organic heterocyclic compounds containing a six-membered saturated heterocycle with two nitrogen, one sulfur, and three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thiadiazinanes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Thiadiazinanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiadiazinane
- Cyclic dithiocarbamic acid ester
- Dithiocarbamic acid ester
- Azacycle
- Hemithioaminal
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organosulfur compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03fv-9800000000-aa787fe0c30c555215f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0udi-9000000000-bf9941b6e7a6373b7f2c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0udi-9000000000-368c42a6d7ee85141a77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-3ecedce2ba244c2f12de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-9500000000-f738742d8c3ded2dfadd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00fu-9100000000-2f83da49ca198d71f490 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1900000000-3e8a6f9481c91dfc61ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-6562c489ef7514276a00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-7a22a443ad1ae1644cf8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-17273c55bad7b32a733a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-3900000000-819e429fabd42676a5c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-9000000000-2715701f2952b363f29c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-2900000000-114e0dedaeab968a6e69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9200000000-68336301c10a101a4177 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9100000000-1b39126daf8886f95467 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9100000000-5a7d67bda2e5c3972d54 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dazomet |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 75212 |
|---|
| PubChem Compound ID | 10788 |
|---|
| Kegg Compound ID | C18457 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|