| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:50 UTC |
|---|
| Update Date | 2016-11-09 01:08:58 UTC |
|---|
| Accession Number | CHEM002757 |
|---|
| Identification |
|---|
| Common Name | Benfluralin |
|---|
| Class | Small Molecule |
|---|
| Description | Benfluralin is an herbicide of the dinitroaniline class. It is used to control grasses and other weeds. Annual use in the United States was approximately 700,000 pounds in 2004. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- EPA Endocrine Screening
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Herbicide
- Organic Compound
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
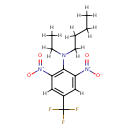
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha,alpha,alpha-Trifluoro-2,6-dinitro-N,N-ethylbutyl-p-toluidine | ChEBI | | Benefin | ChEBI | | Benfluraline | ChEBI | | Bethrodine | ChEBI | | EL-110 | ChEBI | | N-Butyl-N-ethyl-2,6-dinitro-4-(trifluoromethyl)benzenamine | ChEBI | | N-Butyl-N-ethyl-alpha,alpha,alpha-trifluoro-2,6-dinitro-p-toluidine | ChEBI | | a,a,a-Trifluoro-2,6-dinitro-N,N-ethylbutyl-p-toluidine | Generator | | Α,α,α-trifluoro-2,6-dinitro-N,N-ethylbutyl-p-toluidine | Generator | | N-Butyl-N-ethyl-a,a,a-trifluoro-2,6-dinitro-p-toluidine | Generator | | N-Butyl-N-ethyl-α,α,α-trifluoro-2,6-dinitro-p-toluidine | Generator | | Balan | MeSH |
|
|---|
| Chemical Formula | C13H16F3N3O4 |
|---|
| Average Molecular Mass | 335.279 g/mol |
|---|
| Monoisotopic Mass | 335.109 g/mol |
|---|
| CAS Registry Number | 1861-40-1 |
|---|
| IUPAC Name | N-butyl-N-ethyl-2,6-dinitro-4-(trifluoromethyl)aniline |
|---|
| Traditional Name | benefin |
|---|
| SMILES | [H]C1=C(C(N(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])=C(C([H])=C1C(F)(F)F)[N+]([O-])=O)[N+]([O-])=O |
|---|
| InChI Identifier | InChI=1S/C13H16F3N3O4/c1-3-5-6-17(4-2)12-10(18(20)21)7-9(13(14,15)16)8-11(12)19(22)23/h7-8H,3-6H2,1-2H3 |
|---|
| InChI Key | SMDHCQAYESWHAE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dinitroanilines. These are organic compounds containing an aniline moiety, which is substituted at 2 positions by a nitro group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Aniline and substituted anilines |
|---|
| Direct Parent | Dinitroanilines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dinitroaniline
- Trifluoromethylbenzene
- Nitrobenzene
- Nitroaromatic compound
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- C-nitro compound
- Tertiary amine
- Organic nitro compound
- Organic oxoazanium
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Alkyl fluoride
- Organic zwitterion
- Hydrocarbon derivative
- Organic oxide
- Organonitrogen compound
- Organofluoride
- Organopnictogen compound
- Organohalogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Alkyl halide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 65.0°C | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0adi-4189000000-455a47c7acbdb603b982 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1009000000-059ed03818ef425cb302 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06vi-3019000000-2d0b0fea633d7772646a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-3ff165b76f5154399619 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-e9e44e7c0e33354b601d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1009000000-571f5778b80506bfeec9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9043000000-023c514bbf6ee9857aab | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Benfluralin has shown to be toxic to the kidneys, liver and thyroid in long-term animal studies. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0248950 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Benfluralin |
|---|
| Chemspider ID | 2229 |
|---|
| ChEBI ID | 132878 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|