| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2013-04-25 07:56:50 UTC |
|---|
| Update Date | 2016-11-09 01:08:58 UTC |
|---|
| Accession Number | CHEM002754 |
|---|
| Identification |
|---|
| Common Name | Anilazine |
|---|
| Class | Small Molecule |
|---|
| Description | Anilazine (ǎ-nǐl-a-zēn) is an organic compound with the chemical formula C9H5Cl3N4. It is a pesticide used on crops. It comes under the category of triazine fungicides. It is used for controlling fungus diseases which attack lawns and turf, cereals, coffee, and a wide variety of vegetables and other crops. It is also used for the control of potato and tomato leafspots. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Fungicide
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
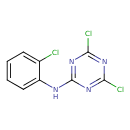
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (O-Chloroanilino)dichlorotriazine | ChEBI | | 2,4-Dichloro-6-(2-chloroanilino)-1,3,5-triazine | ChEBI | | 2,4-Dichloro-6-(O-chloroanilino)-S-triazine | ChEBI | | 2-(2-Chloranilin)-4,6-dichlor-1,3,5-triazin | ChEBI | | 2-Chloro-N-(4,6-dichloro-1,3,5-triazin-2-yl)aniline | ChEBI | | Anilazin | ChEBI | | Aniyaline | ChEBI | | Dairene | ChEBI | | Dyrene | ChEBI | | Kemate | ChEBI | | Triasym | ChEBI | | Triasyn | ChEBI | | Zinochlor | ChEBI | | Kemic acid | Generator |
|
|---|
| Chemical Formula | C9H5Cl3N4 |
|---|
| Average Molecular Mass | 275.522 g/mol |
|---|
| Monoisotopic Mass | 273.958 g/mol |
|---|
| CAS Registry Number | 101-05-3 |
|---|
| IUPAC Name | 4,6-dichloro-N-(2-chlorophenyl)-1,3,5-triazin-2-amine |
|---|
| Traditional Name | anilazine |
|---|
| SMILES | ClC1=CC=CC=C1NC1=NC(Cl)=NC(Cl)=N1 |
|---|
| InChI Identifier | InChI=1S/C9H5Cl3N4/c10-5-3-1-2-4-6(5)13-9-15-7(11)14-8(12)16-9/h1-4H,(H,13,14,15,16) |
|---|
| InChI Key | IMHBYKMAHXWHRP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aniline and substituted anilines. These are organic compounds containing an aminobenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Aniline and substituted anilines |
|---|
| Direct Parent | Aniline and substituted anilines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aniline or substituted anilines
- Amino-1,3,5-triazine
- Chloro-s-triazine
- Halo-s-triazine
- Aminotriazine
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- 1,3,5-triazine
- Triazine
- Heteroaromatic compound
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organohalogen compound
- Organochloride
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-024i-5190000000-096b2b160cf27828672d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-46bf0b5a9300e8e76de8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-fc253cf4588f35943ec3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03ds-9520000000-67d4f3fd5cca845ac51f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03k9-0490000000-9cdec0b6ad8cab98428a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0190000000-c200c1607added010fbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007a-1940000000-d8df9ea97e9ebcbbe4b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-8005ca7887feae9d66ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-8005ca7887feae9d66ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w29-2970000000-796603edc674fcb6c058 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-2375465bd2ec3079551c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0190000000-ca2748b9a77e9fa8ecbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1900000000-c660f45513d52eb02fae | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-000l-9460000000-49e6337d109dc544f2a7 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral
Dermal
Intraperitoneal. |
|---|
| Mechanism of Toxicity | Anilazine was found to be mutagenic. |
|---|
| Metabolism | Metabolites of amilazine can be found in the urine and the feces.The major identified metabolite is the hydroxylation product, 2-(2-chloroanilino)-s-triazinodione, is mosltly present in the urine. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | LD50: 2710 mg/kg (rats), 400 mg/kg (rabbits) |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Can cause dermatitis, irritation of eyes and respiratory tract. |
|---|
| Symptoms | Diarrhea; vomitting
|
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0248437 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Anilazine |
|---|
| Chemspider ID | 7260 |
|---|
| ChEBI ID | 82076 |
|---|
| PubChem Compound ID | 7541 |
|---|
| Kegg Compound ID | C18935 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|