| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-23 18:26:07 UTC |
|---|
| Update Date | 2016-11-09 01:08:46 UTC |
|---|
| Accession Number | CHEM002419 |
|---|
| Identification |
|---|
| Common Name | beta-Methylamino-L-alanine |
|---|
| Class | Small Molecule |
|---|
| Description | Methylamino-L-alanine,КBMAA, is a non-proteinogenic amino acid produced by cyanobacteria. It is a plant toxin found in the seeds of the cycad (division Cycadophyta). It is produced by cyanobacteria of the genus Nostoc that live on the plant's roots and exibits neurotoxic effects. Acutely, BMAA can act as an excitotoxin on glutamate receptors such as NMDA, calcium dependent AMPA and kainite receptors. The activation of the metabotropic glutamate receptor 5 is believed to induce oxidative stress in the neuron by depletion of glutathione. BMAA is also suggested to misincorporate into nascent proteins in place of L-Serine, possibly causing protein misfolding and aggregation, both hallmarks of tangle diseases, including Alzheimer's, Parkinson's and ALS, PSP and Lewy Body Disease. In-vitro research has shown that protein association of L-BMAA can be inhibited in presence of excess L-Serine. (Wikipedia) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | - Amine
- Bacterial Toxin
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
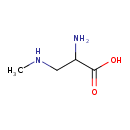
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-Methylamino-L-alanine | Generator | | Β-methylamino-L-alanine | Generator | | beta-N-methylamino-L-Alanine | MeSH | | 2-amino-3(methylamino)Propionic acid | MeSH | | alpha-amino-beta-Methylaminopropionate | MeSH | | L-BMAA | MeSH |
|
|---|
| Chemical Formula | C4H10N2O2 |
|---|
| Average Molecular Mass | 118.134 g/mol |
|---|
| Monoisotopic Mass | 118.074 g/mol |
|---|
| CAS Registry Number | 15920-93-1 |
|---|
| IUPAC Name | 2-amino-3-(methylamino)propanoic acid |
|---|
| Traditional Name | 3-(methylamino)-(DL)-alanine |
|---|
| SMILES | CNCC(N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H10N2O2/c1-6-2-3(5)4(7)8/h3,6H,2,5H2,1H3,(H,7,8) |
|---|
| InChI Key | UJVHVMNGOZXSOZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Amino acid
- Carboxylic acid
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Secondary amine
- Carbonyl group
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-723c43f86fac93138d59 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00rf-9300000000-e8cc67e99f8392f03079 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-2a99e5543703f59a60b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-45a7801287a3f96b30d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2900000000-f1d1f230db250f166707 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9700000000-a72edffd3071d783a73e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007x-9000000000-0330bca304c61e32681b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xr-8900000000-47a4739a7db5fa52bfc1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-9000000000-0657b74281268f49b22e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-942f1b1aaa14f67379b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-76f78092b136dbfaef3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-6900000000-913082641e0856b80db9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-8b5a890d1e3276284ef9 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (ingestion) (2) ; dermal (2) |
|---|
| Mechanism of Toxicity | BMAA may cause neurotoxic effects by damaging cells (such as neurons) in the motor cortex and spinal cord. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Methylamino-L-alanine, beta- is a plant toxin found in the seeds of the cycad (division Cycadophyta). (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | BMAA is considered a possible cause of the amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC). Its neurotoxic effects may include degenerative locomotor diseases resulting from damage to cells (such as neurons) in the motor cortex and spinal cord. (1) |
|---|
| Symptoms | Symptoms of BMAA exposure may include limb muscle atrophy and behavioral dysfunction. (1) |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0244715 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Beta-Methylamino-L-alanine |
|---|
| Chemspider ID | 26564 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|