| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:28:37 UTC |
|---|
| Update Date | 2016-11-09 01:08:45 UTC |
|---|
| Accession Number | CHEM002375 |
|---|
| Identification |
|---|
| Common Name | Rifapentine |
|---|
| Class | Small Molecule |
|---|
| Description | Rifapentine is only found in individuals that have used or taken this drug. It is an antibiotic drug used in the treatment of tuberculosis. Rifapentine has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages. Rifapentine inhibits DNA-dependent RNA polymerase in susceptible strains of M. tuberculosis. Rifapentine acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Antibiotic, Antitubercular
- Antimycobacterial
- Drug
- Ester
- Ether
- Hydrazine
- Leprostatic Agent
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
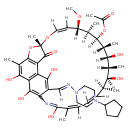
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C47H64N4O12 |
|---|
| Average Molecular Mass | 877.031 g/mol |
|---|
| Monoisotopic Mass | 876.452 g/mol |
|---|
| CAS Registry Number | 61379-65-5 |
|---|
| IUPAC Name | (7S,9Z,11S,12R,13S,14R,15R,16R,17S,18S,21Z)-26-[(1E)-[(4-cyclopentylpiperazin-1-yl)imino]methyl]-2,15,17,23,27,29-hexahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6-oxo-8,30-dioxa-24-azatetracyclo[23.3.1.1^{4,7}.0^{5,28}]triaconta-1(28),2,4,9,19,21,23,25(29),26-nonaen-13-yl acetate |
|---|
| Traditional Name | (7S,9Z,11S,12R,13S,14R,15R,16R,17S,18S,21Z)-26-[(1E)-[(4-cyclopentylpiperazin-1-yl)imino]methyl]-2,15,17,23,27,29-hexahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6-oxo-8,30-dioxa-24-azatetracyclo[23.3.1.1^{4,7}.0^{5,28}]triaconta-1(28),2,4,9,19,21,23,25(29),26-nonaen-13-yl acetate |
|---|
| SMILES | [H]\C(=N/N1CCN(CC1)C1CCCC1)C1=C(O)C2=C3C(O)=C(C)C4=C2C(=O)[C@](C)(O4)O\C([H])=C([H])/[C@]([H])(OC)[C@@]([H])(C)[C@@]([H])(OC(C)=O)[C@]([H])(C)[C@]([H])(O)[C@]([H])(C)[C@@]([H])(O)[C@@]([H])(C)C([H])=C([H])\C([H])=C(C)/C(O)=NC1=C3O |
|---|
| InChI Identifier | InChI=1S/C47H64N4O12/c1-24-13-12-14-25(2)46(59)49-37-32(23-48-51-20-18-50(19-21-51)31-15-10-11-16-31)41(56)34-35(42(37)57)40(55)29(6)44-36(34)45(58)47(8,63-44)61-22-17-33(60-9)26(3)43(62-30(7)52)28(5)39(54)27(4)38(24)53/h12-14,17,22-24,26-28,31,33,38-39,43,53-57H,10-11,15-16,18-21H2,1-9H3,(H,49,59)/b13-12+,22-17-,25-14-,48-23+/t24-,26+,27+,28+,33-,38-,39+,43+,47-/m0/s1 |
|---|
| InChI Key | WDZCUPBHRAEYDL-IWVAIHGYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 2.13e-02 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0aor-0300000190-d118c1ac8e3c60e6452b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-2100002290-b441d6ad4afc88a62624 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9700006020-ee8161658ea49259d168 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0100001290-6aa51e29ae4edc2dea4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-2500003190-ac076b0cb13b991b003a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-9200008320-465071920392bca6a779 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0000000390-f812402cd842a853749c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0690-0000000190-f6ddb4327cad4885918e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cfr-7600001490-17f0e57624f1de4d2dee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1000000290-3b4cef67ccc26d401a39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000010-4e966171b9c3614260a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4m-9500006520-7a1bb51257442c63c784 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly and well absorbed from the gastrointestinal tract. |
|---|
| Mechanism of Toxicity | Rifapentine has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages. Rifapentine inhibits DNA-dependent RNA polymerase in susceptible strains of M. tuberculosis. Rifapentine acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death. |
|---|
| Metabolism | Hepatic
Route of Elimination: Following a single 600 mg oral dose of radiolabeled rifapentine to healthy volunteers (n=4), 87% of the total 14C rifapentine was recovered in the urine (17%) and feces (70%). |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of pulmonary tuberculosis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0015332 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Rifapentine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|