| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:27:59 UTC |
|---|
| Update Date | 2016-11-09 01:08:44 UTC |
|---|
| Accession Number | CHEM002304 |
|---|
| Identification |
|---|
| Common Name | Mesoridazine |
|---|
| Class | Small Molecule |
|---|
| Description | Mesoridazine is only found in individuals that have used or taken this drug. It is a phenothiazine antipsychotic with effects similar to chlorpromazine. [PubChem]Based upon animal studies, mesoridazine, as with other phenothiazines, acts indirectly on reticular formation, whereby neuronal activity into reticular formation is reduced without affecting its intrinsic ability to activate the cerebral cortex. In addition, the phenothiazines exhibit at least part of their activities through depression of hypothalamic centers. Neurochemically, the phenothiazines are thought to exert their effects by a central adrenergic blocking action. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Antipsychotic Agent
- Dopamine Antagonist
- Drug
- Ether
- Metabolite
- Organic Compound
- Phenothiazine
- Synthetic Compound
|
|---|
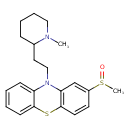
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 10-(2(1-Methyl-2-piperidyl)ethyl)-2-(methylsulfinyl)phenothiazine | ChEBI | | 10-(2-(1-Methyl-2-piperidyl)ethyl)-2-methylsulfinyl phenothiazine | ChEBI | | 2-Methanesulfinyl-10-[2-(1-methyl-piperidin-2-yl)-ethyl]-10H-phenothiazine | ChEBI | | Mesoridazina | ChEBI | | Mesoridazinum | ChEBI | | Thioridazine thiomethyl sulfoxide | ChEBI | | Thioridazine-2-sulfoxide | ChEBI | | TPS-23 | ChEBI | | Lidanar | Kegg | | 10-(2(1-Methyl-2-piperidyl)ethyl)-2-(methylsulphinyl)phenothiazine | Generator | | 10-(2-(1-Methyl-2-piperidyl)ethyl)-2-methylsulphinyl phenothiazine | Generator | | 2-Methanesulphinyl-10-[2-(1-methyl-piperidin-2-yl)-ethyl]-10H-phenothiazine | Generator | | Thioridazine thiomethyl sulphoxide | Generator | | Thioridazine-2-sulphoxide | Generator | | Thioridazien thiomethyl sulfoxide | HMDB | | Thioridazine monosulfoxide analog | HMDB | | TPS23 | HMDB | | Serentil | HMDB |
|
|---|
| Chemical Formula | C21H26N2OS2 |
|---|
| Average Molecular Mass | 386.574 g/mol |
|---|
| Monoisotopic Mass | 386.149 g/mol |
|---|
| CAS Registry Number | 5588-33-0 |
|---|
| IUPAC Name | 2-methanesulfinyl-10-[2-(1-methylpiperidin-2-yl)ethyl]-10H-phenothiazine |
|---|
| Traditional Name | mesoridazine |
|---|
| SMILES | CN1CCCCC1CCN1C2=CC=CC=C2SC2=C1C=C(C=C2)S(C)=O |
|---|
| InChI Identifier | InChI=1S/C21H26N2OS2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(26(2)24)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 |
|---|
| InChI Key | SLVMESMUVMCQIY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenothiazines. These are polycyclic aromatic compounds containing a phenothiazine moiety, which is a linear tricyclic system that consists of a two benzene rings joined by a para-thiazine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiazines |
|---|
| Sub Class | Phenothiazines |
|---|
| Direct Parent | Phenothiazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenothiazine
- Alkyldiarylamine
- Diarylthioether
- Aryl thioether
- Tertiary aliphatic/aromatic amine
- Para-thiazine
- Piperidine
- Benzenoid
- Sulfoxide
- Tertiary amine
- Tertiary aliphatic amine
- Thioether
- Sulfinyl compound
- Azacycle
- Amine
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organosulfur compound
- Organonitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 7.67e-02 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006t-9113000000-c43ca99a239d882aaec0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000i-0119000000-fffe496e2ff640e0c935 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-009j-4409000000-1ac2768cf2aedf0c606d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0109000000-34ffca607374cc2d3dad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002r-3419000000-8395b65edb5ff6d5cb3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-8912000000-af14b6ff98df3cb285e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-2009000000-a670d5fab14237f10c3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-4195000000-c1fd7e13d41cc4d31064 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9320000000-f0c0d591d683e0031797 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-ea6eb7f211262d8b7c60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-5419000000-67a5e726504808a7f165 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0032-9342000000-55bdb60e5caa2bf4c40c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-ae73409bcb6436484a74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0039000000-5b62f5f508c6b499a5f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-9082000000-3b94eddc2f178347c05e | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0002-9331000000-8e14cb597ee411174c3e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral; Intramuscular injection. Well absorbed from the gastrointestinal tract. |
|---|
| Mechanism of Toxicity | Based upon animal studies, mesoridazine, as with other phenothiazines, acts indirectly on reticular formation, whereby neuronal activity into reticular formation is reduced without affecting its intrinsic ability to activate the cerebral cortex. In addition, the phenothiazines exhibit at least part of their activities through depression of hypothalamic centers. Neurochemically, the phenothiazines are thought to exert their effects by a central adrenergic blocking action. |
|---|
| Metabolism |
Half Life: 24 to 48 hours |
|---|
| Toxicity Values | Oral LD50 is 560 ± 62.5 mg/kg and 644 ± 48 mg/kg in mouse and rat, respectively. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in the treatment of schizophrenia, organic brain disorders, alcoholism and psychoneuroses. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose may include emesis, muscle tremors, decreased food intake and death associated with aspiration of oral-gastric contents into the respiratory system. |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00933 |
|---|
| HMDB ID | HMDB0015068 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Mesoridazine |
|---|
| Chemspider ID | 3936 |
|---|
| ChEBI ID | 6780 |
|---|
| PubChem Compound ID | 4078 |
|---|
| Kegg Compound ID | C07143 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|