| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:27:39 UTC |
|---|
| Update Date | 2016-11-09 01:08:43 UTC |
|---|
| Accession Number | CHEM002273 |
|---|
| Identification |
|---|
| Common Name | Sulfasalazine |
|---|
| Class | Small Molecule |
|---|
| Description | Sulfasalazine is only found in individuals that have used or taken this drug. It is a drug that is used in the management of inflammatory bowel diseases. Its activity is generally considered to lie in its metabolic breakdown product, 5-aminosalicylic acid (see mesalamine) released in the colon. (From Martindale, The Extra Pharmacopoeia, 30th ed, p907)The mode of action of Sulfasalazine or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), is still under investigation, but may be related to the anti-inflammatory and/or immunomodulatory properties that have been observed in animal and in vitro models, to its affinity for connective tissue, and/or to the relatively high concentration it reaches in serous fluids, the liver and intestinal walls, as demonstrated in autoradiographic studies in animals. In ulcerative colitis, clinical studies utilizing rectal administration of Sulfasalazine, SP and 5-ASA have indicated that the major therapeutic action may reside in the 5-ASA moiety. The relative contribution of the parent drug and the major metabolites in rheumatoid arthritis is unknown. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- IARC Carcinogens Group 2B
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Anti-Infective Agent
- Anti-Inflammatory Agent, Non-Steroidal
- Antirheumatic Agent
- Drug
- Gastrointestinal Agent
- Metabolite
- Organic Compound
- Sulfonamide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)benzoic acid | ChEBI | | 2-Hydroxy-5-[4-(pyridin-2-ylsulfamoyl)-phenylazo]-benzoic acid | ChEBI | | 4-(Pyridyl-2-amidosulfonyl)-3'-carboxy-4'-hydroxyazobenzene | ChEBI | | 5-((p-(2-Pyridylsulfamoyl)phenyl)azo)salicylic acid | ChEBI | | 5-(4-(2-Pyridylsulfamoyl)phenylazo)-2-hydroxybenzoic acid | ChEBI | | 5-(p-(2-Pyridylsulfamyl)phenylazo)salicylic acid | ChEBI | | Azulfidine | ChEBI | | Salazosulfapiridina | ChEBI | | Salazosulfapyridine | ChEBI | | Salazosulfapyridinum | ChEBI | | Salicylazosulfapyridine | ChEBI | | Sulfasalazina | ChEBI | | Sulfasalazinum | ChEBI | | 2-Hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)benzoate | Generator | | 2-Hydroxy-5-((4-((2-pyridinylamino)sulphonyl)phenyl)azo)benzoate | Generator | | 2-Hydroxy-5-((4-((2-pyridinylamino)sulphonyl)phenyl)azo)benzoic acid | Generator | | 2-Hydroxy-5-[4-(pyridin-2-ylsulfamoyl)-phenylazo]-benzoate | Generator | | 2-Hydroxy-5-[4-(pyridin-2-ylsulphamoyl)-phenylazo]-benzoate | Generator | | 2-Hydroxy-5-[4-(pyridin-2-ylsulphamoyl)-phenylazo]-benzoic acid | Generator | | 4-(Pyridyl-2-amidosulphonyl)-3'-carboxy-4'-hydroxyazobenzene | Generator | | 5-((p-(2-Pyridylsulfamoyl)phenyl)azo)salicylate | Generator | | 5-((p-(2-Pyridylsulphamoyl)phenyl)azo)salicylate | Generator | | 5-((p-(2-Pyridylsulphamoyl)phenyl)azo)salicylic acid | Generator | | 5-(4-(2-Pyridylsulfamoyl)phenylazo)-2-hydroxybenzoate | Generator | | 5-(4-(2-Pyridylsulphamoyl)phenylazo)-2-hydroxybenzoate | Generator | | 5-(4-(2-Pyridylsulphamoyl)phenylazo)-2-hydroxybenzoic acid | Generator | | 5-(p-(2-Pyridylsulfamyl)phenylazo)salicylate | Generator | | 5-(p-(2-Pyridylsulphamyl)phenylazo)salicylate | Generator | | 5-(p-(2-Pyridylsulphamyl)phenylazo)salicylic acid | Generator | | Salazosulphapiridina | Generator | | Salazosulphapyridine | Generator | | Salazosulphapyridinum | Generator | | Salicylazosulphapyridine | Generator | | Sulphasalazina | Generator | | Sulphasalazinum | Generator | | Sulphasalazine | Generator | | Sulfasalazin | HMDB |
|
|---|
| Chemical Formula | C18H14N4O5S |
|---|
| Average Molecular Mass | 398.393 g/mol |
|---|
| Monoisotopic Mass | 398.068 g/mol |
|---|
| CAS Registry Number | 599-79-1 |
|---|
| IUPAC Name | 2-hydroxy-5-[(E)-2-{4-[(pyridin-2-yl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid |
|---|
| Traditional Name | sulfasalazine |
|---|
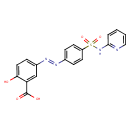
| SMILES | OC(=O)C1=CC(=CC=C1O)\N=N\C1=CC=C(C=C1)S(=O)(=O)NC1=NC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25)/b21-20+ |
|---|
| InChI Key | NCEXYHBECQHGNR-QZQOTICOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as azobenzenes. These are organonitrogen aromatic compounds that contain a central azo group, where each nitrogen atom is conjugated to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azobenzenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Azobenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Azobenzene
- Benzenesulfonamide
- Hydroxybenzoic acid
- Salicylic acid or derivatives
- Salicylic acid
- Benzoic acid or derivatives
- Benzoic acid
- Benzenesulfonyl group
- Benzoyl
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Pyridine
- Imidolactam
- Benzenoid
- Organosulfonic acid amide
- Heteroaromatic compound
- Vinylogous acid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Azo compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 220 dec°C | | Boiling Point | Not Available | | Solubility | 4.64e-02 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f6w-6695000000-47b5c3a0d5190268e477 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-2549110000-cce76e754cd947c9bdf8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0002-0239100000-5bf223e6e250eb77105a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-9e5b613387b866753d70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-2019000000-5dbdcf5699c56f6a59d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002b-9240000000-6268787fab73d296c1d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0009000000-5eea07137968cfa96d18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-642c65b2544ce824decc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k97-9663000000-5acc71d633a9ef82ea98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-a266ecec49bba90668ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-005a-2089000000-ad13a0c69c0256eb5505 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0092-3936000000-d0a391d2c705ca56748e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-cf36a51de9de200e8014 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2917000000-1f1274dcd32e5287cfb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-1900000000-16464eaa00e58b69be2d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral |
|---|
| Mechanism of Toxicity | The mode of action of Sulfasalazine or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), is still under investigation, but may be related to the anti-inflammatory and/or immunomodulatory properties that have been observed in animal and in vitro models, to its affinity for connective tissue, and/or to the relatively high concentration it reaches in serous fluids, the liver and intestinal walls, as demonstrated in autoradiographic studies in animals. In ulcerative colitis, clinical studies utilizing rectal administration of Sulfasalazine, SP and 5-ASA have indicated that the major therapeutic action may reside in the 5-ASA moiety. The relative contribution of the parent drug and the major metabolites in rheumatoid arthritis is unknown. |
|---|
| Metabolism |
Route of Elimination: The majority of 5-ASA stays within the colonic lumen and is excreted as 5-ASA and acetyl-5-ASA with the feces.
Half Life: 5-10 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (3) |
|---|
| Uses/Sources | Used primarily as an anti-inflammatory agent in the treatment of inflammatory bowel disease as well as for rheumatoid arthritis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Sulfasalazine in rare cases can cause severe depression in young males. Immune thrombocytopenia has been reported. [Wikipedia] |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Gastric lavage or emesis plus catharsis as indicated. Alkalinize urine. If kidney function is normal, force fluids. If anuria is present, restrict fluids and salt, and treat appropriately. Catheterization of the ureters may be indicated for complete renal blockage by crystals. The low molecular weight of sulfasalazine and its metabolites may facilitate their removal by dialysis. (2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00795 |
|---|
| HMDB ID | HMDB0014933 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sulfasalazine |
|---|
| Chemspider ID | 10605946 |
|---|
| ChEBI ID | 9334 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C07316 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | |
|---|