| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:27:35 UTC |
|---|
| Update Date | 2016-11-09 01:08:43 UTC |
|---|
| Accession Number | CHEM002266 |
|---|
| Identification |
|---|
| Common Name | Isoflurane |
|---|
| Class | Small Molecule |
|---|
| Description | Isoflurane is only found in individuals that have used or taken this drug. It is a stable, non-explosive inhalation anesthetic, relatively free from significant side effects. [PubChem]Isoflurane induces a reduction in junctional conductance by decreasing gap junction channel opening times and increasing gap junction channel closing times. Isoflurane also activates calcium dependent ATPase in the sarcoplasmic reticulum by increasing the fluidity of the lipid membrane. Also appears to bind the D subunit of ATP synthase and NADH dehydogenase. Isoflurane also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor and the glycine receptor. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- HPV EPA Chemicals
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Anesthetic
- Anesthetic, Inhalation
- Drug
- General Anesthetic
- Household Toxin
- Metabolite
- Organic Compound
- Organochloride
- Organofluoride
- Synthetic Compound
|
|---|
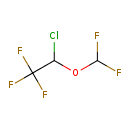
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Chloro-2,2,2-trifluoroethyl difluoromethyl ether | ChEBI | | Aerrane | ChEBI | | Ethane | ChEBI | | Forane | ChEBI | | Forene | ChEBI | | Isoflurano | ChEBI | | Isofluranum | ChEBI |
|
|---|
| Chemical Formula | C3H2ClF5O |
|---|
| Average Molecular Mass | 184.492 g/mol |
|---|
| Monoisotopic Mass | 183.971 g/mol |
|---|
| CAS Registry Number | 26675-46-7 |
|---|
| IUPAC Name | 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane |
|---|
| Traditional Name | isoflurane |
|---|
| SMILES | FC(F)OC(Cl)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C3H2ClF5O/c4-1(3(7,8)9)10-2(5)6/h1-2H |
|---|
| InChI Key | PIWKPBJCKXDKJR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organofluorides. Organofluorides are compounds containing a chemical bond between a carbon atom and a fluorine atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Organofluorides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Organofluorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organofluoride
- Organochloride
- Alkyl halide
- Alkyl fluoride
- Alkyl chloride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 48-48.5°C | | Boiling Point | 48.5°C | | Solubility | 4470 mg/L (at 37°C) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-9400000000-6ed40f9f8dd595d42648 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-86cdc849d98cebceb41f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-638085524e003bcf4003 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9400000000-d8b25b66c3eceff9f5ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-ebe1660e853c066c6392 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-0900000000-30bbfd349d22f8057b18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-4900000000-0717c6cbf8950b9de9d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-f72d585725966be19d66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-db40d4801d1fbe19bc48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-1900000000-8de2342c64e7c06ad3bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-cda3ea1a89b8c052a64a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000000-cda3ea1a89b8c052a64a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-1900000000-90e8f4a64ee24b4cbf9b | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0udi-9300000000-c134e176400bbe7073c2 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation |
|---|
| Mechanism of Toxicity | Isoflurane induces a reduction in junctional conductance by decreasing gap junction channel opening times and increasing gap junction channel closing times. Isoflurane also activates calcium dependent ATPase in the sarcoplasmic reticulum by increasing the fluidity of the lipid membrane. Also appears to bind the D subunit of ATP synthase and NADH dehydogenase. Isoflurane also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor and the glycine receptor. |
|---|
| Metabolism | Minimal |
|---|
| Toxicity Values | LC50=15300 ppm/3 hrs (inhalation by rat) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For induction and maintenance of general anesthesia. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | May lead to cardiac arrhythmias and death (Rarely). In susceptible individuals, Isoflurane anesthesia may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. The syndrome includes nonspecific features such as muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias, and unstable blood pressure.(1) |
|---|
| Symptoms | The predicted effects of acute overexposure by inhalation of Isoflurane, USP include headache, dizziness or (in extreme cases) unconsciousness. (1) |
|---|
| Treatment | Stop drug administration, establish a clear airway, and initiate assisted or controlled ventilation with pure oxygen. (2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00753 |
|---|
| HMDB ID | HMDB0014891 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Isoflurane |
|---|
| Chemspider ID | 3631 |
|---|
| ChEBI ID | 6015 |
|---|
| PubChem Compound ID | 3763 |
|---|
| Kegg Compound ID | C07518 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Leonid A. Rozov, Fernando Quiroz, Gerald G. Vernice, “Preparation of isoflurane.” U.S. Patent US5416244, issued March, 1973. |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|