| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:27:27 UTC |
|---|
| Update Date | 2016-11-09 01:08:43 UTC |
|---|
| Accession Number | CHEM002251 |
|---|
| Identification |
|---|
| Common Name | Amprenavir |
|---|
| Class | Small Molecule |
|---|
| Description | Amprenavir is only found in individuals that have used or taken this drug. It is a protease inhibitor used to treat HIV infection.Amprenavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amide
- Amine
- Anti-HIV Agent
- Antibiotic, Antitubercular
- Drug
- Ester
- Ether
- HIV Protease Inhibitor
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
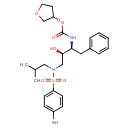
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3S)-Tetrahydro-3-furanyl ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamate | ChEBI | | Agenerase | ChEBI | | (3S)-Tetrahydro-3-furanyl ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamic acid | Generator | | (3S)-Tetrahydro-3-furanyl ((1S,2R)-3-(((4-aminophenyl)sulphonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamate | Generator | | (3S)-Tetrahydro-3-furanyl ((1S,2R)-3-(((4-aminophenyl)sulphonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamic acid | Generator | | AMP | HMDB | | AMV | HMDB | | VX-478 | HMDB | | GlaxoSmithKline brand OF amprenavir | HMDB | | Vertex VX478 | HMDB | | Glaxo wellcome brand OF amprenavir | HMDB | | Tetrahydro-3-furyl N-(3-(4-amino-N-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxypropyl)carbamate | HMDB |
|
|---|
| Chemical Formula | C25H35N3O6S |
|---|
| Average Molecular Mass | 505.627 g/mol |
|---|
| Monoisotopic Mass | 505.225 g/mol |
|---|
| CAS Registry Number | 161814-49-9 |
|---|
| IUPAC Name | (3S)-oxolan-3-yl N-[(2S,3R)-3-hydroxy-4-[N-(2-methylpropyl)4-aminobenzenesulfonamido]-1-phenylbutan-2-yl]carbamate |
|---|
| Traditional Name | amprenavir |
|---|
| SMILES | CC(C)CN(C[C@@H](O)[C@H](CC1=CC=CC=C1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)C1=CC=C(N)C=C1 |
|---|
| InChI Identifier | InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 |
|---|
| InChI Key | YMARZQAQMVYCKC-OEMFJLHTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aminobenzenesulfonamides. These are organic compounds containing a benzenesulfonamide moiety with an amine group attached to the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Aminobenzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzenesulfonamide
- Phenylbutylamine
- Amphetamine or derivatives
- Benzenesulfonyl group
- Aniline or substituted anilines
- Organosulfonic acid amide
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Carbamic acid ester
- Tetrahydrofuran
- Sulfonyl
- Carbonic acid derivative
- Secondary alcohol
- Dialkyl ether
- Ether
- Oxacycle
- Organoheterocyclic compound
- Organic oxide
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Amine
- Alcohol
- Carbonyl group
- Primary amine
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 4.91e-02 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-022d-8491400000-418cbb7f08471fa84619 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-03kc-7192320000-fa264cdbd41779709c30 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-05mk-2692510000-696582f37a165753c2f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-9000500000-e60fa95c31e43c49b031 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9001100000-2c5d3283dd48ceae82dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9010000000-cd15933ef1ecd45d9b1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gbi-4304930000-6cf1884fae8acb8332ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0173-9432500000-a4d70ca4c7ac6bd2930d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9825100000-b5b4efee0204074368f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0046920000-a443e441b8dc951bec99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2922000000-15960e61915142ff5b1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-78c87a49bf59b5f6c014 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uxr-2302980000-1c2dbf56f0de2b3df597 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3911710000-48ad17f577dc556faafc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-7900200000-41ad730c9ba505f33793 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly absorbed after oral administration in HIV-1-infected patients with a time to peak concentration (Tmax) typically between 1 and 2 hours after a single oral dose. The absolute oral bioavailability of amprenavir in humans has not been established. |
|---|
| Mechanism of Toxicity | Amprenavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles. |
|---|
| Metabolism | Hepatic. Amprenavir is metabolized in the liver by the cytochrome P450 3A4 (CYP3A4) enzyme system. The 2 major metabolites result from oxidation of the tetrahydrofuran and aniline moieties. Glucuronide conjugates of oxidized metabolites have been identified as minor metabolites in urine and feces.
Half Life: 7.1-10.6 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of HIV-1 infection in combination with other antiretroviral agents. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | If overdosage occurs, the patient should be monitored for evidence of toxicity and standard supportive treatment applied as necessary. (2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00701 |
|---|
| HMDB ID | HMDB0014839 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | 478 |
|---|
| Wikipedia Link | Amprenavir |
|---|
| Chemspider ID | 58532 |
|---|
| ChEBI ID | 40050 |
|---|
| PubChem Compound ID | 65016 |
|---|
| Kegg Compound ID | C08086 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|