| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:27:06 UTC |
|---|
| Update Date | 2016-11-09 01:08:42 UTC |
|---|
| Accession Number | CHEM002217 |
|---|
| Identification |
|---|
| Common Name | Amoxapine |
|---|
| Class | Small Molecule |
|---|
| Description | Amoxapine, the N-demethylated derivative of the antipsychotic agent loxapine, is a dibenzoxazepine-derivative tricyclic antidepressant (TCA). TCAs are structurally similar to phenothiazines. They contain a tricyclic ring system with an alkyl amine substituent on the central ring. In non-depressed individuals, amoxapine does not affect mood or arousal, but may cause sedation. In depressed individuals, amoxapine exerts a positive effect on mood. TCAs are potent inhibitors of serotonin and norepinephrine reuptake. In addition, TCAs down-regulate cerebral cortical β-adrenergic receptors and sensitize post-synaptic serotonergic receptors with chronic use. The antidepressant effects of TCAs are thought to be due to an overall increase in serotonergic neurotransmission. TCAs also block histamine H1 receptors, α1-adrenergic receptors and muscarinic receptors, which accounts for their sedative, hypotensive and anticholinergic effects (e.g. blurred vision, dry mouth, constipation, urinary retention), respectively. See toxicity section below for a complete listing of side effects. Amoxapine may be used to treat neurotic and reactive depressive disorders, endogenous and psychotic depression, and mixed symptoms of depression and anxiety or agitation. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Adrenergic Uptake Inhibitor
- Amine
- Antidepressant, Second-Generation
- Antidepressive Agent, Second-Generation
- Dopamine Antagonist
- Drug
- Ether
- Metabolite
- Neurotransmitter Uptake Inhibitor
- Organic Compound
- Organochloride
- Serotonin Uptake Inhibitor
- Synthetic Compound
|
|---|
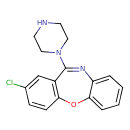
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Chloro-11-(1-piperazinyl)dibenz(b,F)(1,4)oxazepine | ChEBI | | Amoxapina | ChEBI | | Amoxapinum | ChEBI | | Desmethylloxapin | ChEBI | | Asendin | Kegg | | Amoxepine | HMDB | | Amoxapine lederle brand | HMDB | | Défanyl | HMDB | | Cyanamid brand OF amoxapine | HMDB | | Amoxapine wyeth brand | HMDB | | Asendis | HMDB | | Desmethylloxapine | HMDB | | Amoxapine cyanamid brand | HMDB | | Demolox | HMDB | | Lederle brand OF amoxapine | HMDB | | Wyeth brand OF amoxapine | HMDB |
|
|---|
| Chemical Formula | C17H16ClN3O |
|---|
| Average Molecular Mass | 313.781 g/mol |
|---|
| Monoisotopic Mass | 313.098 g/mol |
|---|
| CAS Registry Number | 14028-44-5 |
|---|
| IUPAC Name | 13-chloro-10-(piperazin-1-yl)-2-oxa-9-azatricyclo[9.4.0.0³,⁸]pentadeca-1(11),3,5,7,9,12,14-heptaene |
|---|
| Traditional Name | amoxapine |
|---|
| SMILES | ClC1=CC2=C(OC3=CC=CC=C3N=C2N2CCNCC2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C17H16ClN3O/c18-12-5-6-15-13(11-12)17(21-9-7-19-8-10-21)20-14-3-1-2-4-16(14)22-15/h1-6,11,19H,7-10H2 |
|---|
| InChI Key | QWGDMFLQWFTERH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzoxazepines. Dibenzoxazepines are compounds containing a dibenzoxazepine moiety, which consists of two benzene connected by an oxazepine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzoxazepines |
|---|
| Sub Class | Dibenzoxazepines |
|---|
| Direct Parent | Dibenzoxazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzoxazepine
- Diaryl ether
- Aryl chloride
- Aryl halide
- 1,4-diazinane
- Piperazine
- Imidolactam
- Benzenoid
- Amidine
- Carboxylic acid amidine
- Secondary aliphatic amine
- Oxacycle
- Ether
- Azacycle
- Secondary amine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organic oxygen compound
- Organohalogen compound
- Organochloride
- Organonitrogen compound
- Organooxygen compound
- Amine
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 175-176°C | | Boiling Point | Not Available | | Solubility | 1.71e-01 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-6190000000-1acb98762576326ae75b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-00dl-2690000000-0e598ca107ca857b8f06 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-044i-0169000000-d1665f2e6d27a9efaa39 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-002f-0940000000-9bf5c13f1f2b9b04f465 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00dl-2690000000-0e598ca107ca857b8f06 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-006x-2950000000-2fb725746001d0be41d1 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0006-0910000000-7df25fea8ffb96c66d50 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03k9-1059000000-99ec364d324293ba9191 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0009000000-fb09780a1d33ba8eb3c8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00di-2090000000-046bbbcb38472a6b5a4c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-00di-2290000000-47774f87a43619d76d34 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-00di-2290000000-53b12cd961449d9ea634 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-873be01dd22731e1f7a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0049000000-3989b74ad41770f20ee0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01r6-9440000000-54259557a6e11d912eb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0019000000-eaaa73a51b5bbc0bf28b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0049000000-be3ce6855a4cc094b8fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9250000000-d2f001cf68189e0c19d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-c66d97d82d39ee90b585 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-d2c945077b93a0423349 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00e9-4091000000-4a8bdcd3ca72bcad379c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-06ad1e364d70213564e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0039000000-17396abffdd86c9873e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05e9-1291000000-4d8a424af3692977794b | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-052b-4490000000-6bbd0d2c0c8fe332c636 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, rapidly and almost completely absorbed from the GI tract. Peak plasma concentrations occur within 1-2 hours of oral administration of a single doser. |
|---|
| Mechanism of Toxicity | Amoxapine acts by decreasing the reuptake of norepinephrine and serotonin (5-HT). |
|---|
| Metabolism | Amoxapine is almost completely metabolized in the liver to its major metabolite, 8-hydroxyamoxapine, and a minor metabolite, 7-hydroxyamoxapine. Both metabolites are phamacologically inactive and have half-lives of approximately 30 and 6.5 hours, respectively.
Route of Elimination: 60-69% of a single orally administered dose of amoxapine is excreted in urine, principally as conjugated metabolites. 7-18% of the dose is excrete feces mainly as unconjugated metabolites. Less than 5% of the dose is excreted as unchanged drug in urine.
Half Life: 8 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the relief of symptoms of depression in patients with neurotic or reactive depressive disorders as well as endogenous and psychotic depressions. May also be used to treat depression accompanied by anxiety or agitation. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Toxic manifestations of amoxapine overdosage differ significantly from those of other tricyclic antidepressants. Serious cardiovascular effects are seldom if ever observed. However, CNS effects, particularly grand mal convulsions, occur frequently, and treatment should be directed primarily toward prevention or control of seizures. Status epilepticus may develop and constitutes a neurologic emergency. Coma and acidosis are other serious complications of substantial amoxapine overdosage in some cases. Renal failure may develop two to five days after toxic overdose in patients who may appear otherwise recovered. Acute tubular necrosis with rhabdomuolysis and myolobinurla is the most common renal complication in such cases. This reaction probably occurs in less than 5% of overdose cases, and typically in those who have experienced multiple seizures. |
|---|
| Treatment | Treatment of amoxapine overdosage should be symptomatic and supportive, but with special attention to prevention or control of seizures. If the patient is conscious, induced emesis followed by gastric lavage with appropriate precautions to prevent pulmonary aspiration should be accomplished as soon as possible. Following lavage, activated charcoal may be administered to reduce absorption, and repeated administrations may facilitate drug elimination. An adequate airway should be established in comatose patients and assisted ventilation instituted if necessary. Seizures may respond to standard anticonvulsant therapy such as intravenous diazepam and/or phenytoin. The value of physostigmine appears less certain. Status epilepticus, should it develop, requires vigorous treatment. (2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00543 |
|---|
| HMDB ID | HMDB0014683 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Amoxapine |
|---|
| Chemspider ID | 2085 |
|---|
| ChEBI ID | 2675 |
|---|
| PubChem Compound ID | 2170 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Howell, C.F., Hardy, R.A., Jr. and Quinones, N.Q.; US. Patent 3,663,696; May 16, 1972; assigned to American Cyanamid Company
Howell, C.F., Hardy, R.A., Jr. and Quinones, N.Q.; U.S. Patent 3,681,357; August 1, 1972; assigned to American Cyanamid Company |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|