| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:26:53 UTC |
|---|
| Update Date | 2016-11-09 01:08:42 UTC |
|---|
| Accession Number | CHEM002194 |
|---|
| Identification |
|---|
| Common Name | Prochlorperazine |
|---|
| Class | Small Molecule |
|---|
| Description | Prochlorperazine is only found in individuals that have used or taken this drug. It is a phenothiazine antipsychotic used principally in the treatment of nausea; vomiting; and vertigo. It is more likely than chlorpromazine to cause extrapyramidal disorders. (From Martindale, The Extra Pharmacopoeia, 30th ed, p612) The mechanism of action of prochlorperazine has not been fully determined, but may be primarily related to its antidopaminergic effects. Prochlorperazine blocks the D2 somatodendritic autoreceptor, resulting in the blockade of postsynaptic dopamine receptors in the mesolimbic system and an increased dopamine turnover. Prochlorperazine also has anti-emetic effects, which can be attributed to dopamine blockade in the chemoreceptor trigger zone. Prochlorperazine also blocks anticholinergic and alpha-adrenergic receptors, the blockade of alpha(1)-adrenergic receptors resulting in sedation, muscle relaxation, and hypotension. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Antiemetic
- Antipsychotic Agent
- Dopamine Antagonist
- Drug
- Ether
- Metabolite
- Organic Compound
- Organochloride
- Phenothiazine
- Synthetic Compound
|
|---|
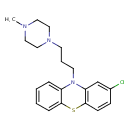
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Chloro-10-(3-(1-methyl-4-piperazinyl)propyl)-phenothiazine | ChEBI | | 2-Chloro-10-(3-(4-methyl-1-piperazinyl)propyl)phenothiazine | ChEBI | | 3-Chloro-10-(3-(1-methyl-4-piperazinyl)propyl)phenothiazine | ChEBI | | 3-Chloro-10-(3-(4-methyl-1-piperazinyl)propyl)phenothiazine | ChEBI | | Chloro-3 (N-methylpiperazinyl-3 propyl)-10 phenothiazine | ChEBI | | N-(gamma-(4'-Methylpiperazinyl-1')propyl)-3-chlorophenothiazine | ChEBI | | Prochlorperazin | ChEBI | | Prochlorperazinum | ChEBI | | Prochlorpermazine | ChEBI | | Prochlorpromazine | ChEBI | | Procloperazine | ChEBI | | Proclorperazina | ChEBI | | Compro | Kegg | | N-(g-(4'-Methylpiperazinyl-1')propyl)-3-chlorophenothiazine | Generator | | N-(Γ-(4'-methylpiperazinyl-1')propyl)-3-chlorophenothiazine | Generator | | Chlormeprazine | HMDB | | Chlorperazine | HMDB | | Prochloroperazine | HMDB | | Prochlorpemazine | HMDB | | Prochlorperazine edisylate | HMDB | | Prochlorperazine maleate | HMDB | | Proclorperazine | HMDB | | Compazine | HMDB | | Edisylate salt, prochlorperazine | HMDB | | Prochlorperazine edisylate salt | HMDB | | Salt, prochlorperazine edisylate | HMDB | | Edisylate, prochlorperazine | HMDB | | Maleate, prochlorperazine | HMDB |
|
|---|
| Chemical Formula | C20H24ClN3S |
|---|
| Average Molecular Mass | 373.943 g/mol |
|---|
| Monoisotopic Mass | 373.138 g/mol |
|---|
| CAS Registry Number | 58-38-8 |
|---|
| IUPAC Name | 2-chloro-10-[3-(4-methylpiperazin-1-yl)propyl]-10H-phenothiazine |
|---|
| Traditional Name | compro |
|---|
| SMILES | CN1CCN(CCCN2C3=CC=CC=C3SC3=C2C=C(Cl)C=C3)CC1 |
|---|
| InChI Identifier | InChI=1S/C20H24ClN3S/c1-22-11-13-23(14-12-22)9-4-10-24-17-5-2-3-6-19(17)25-20-8-7-16(21)15-18(20)24/h2-3,5-8,15H,4,9-14H2,1H3 |
|---|
| InChI Key | WIKYUJGCLQQFNW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenothiazines. These are polycyclic aromatic compounds containing a phenothiazine moiety, which is a linear tricyclic system that consists of a two benzene rings joined by a para-thiazine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiazines |
|---|
| Sub Class | Phenothiazines |
|---|
| Direct Parent | Phenothiazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenothiazine
- Alkyldiarylamine
- Diarylthioether
- Aryl thioether
- Tertiary aliphatic/aromatic amine
- N-alkylpiperazine
- N-methylpiperazine
- Para-thiazine
- Aryl chloride
- Aryl halide
- 1,4-diazinane
- Benzenoid
- Piperazine
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Thioether
- Organic nitrogen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 228°C | | Boiling Point | Not Available | | Solubility | 15 mg/L (at 24°C) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-01vo-9853000000-ba88fedd94e150c34e1a | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0229-7933000000-4e14a9a9728c361941d3 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-01vo-9853000000-ba88fedd94e150c34e1a | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0229-7933000000-4e14a9a9728c361941d3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06xt-9553000000-7c5cfdffb1424ab9b4c3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00fr-0219000000-abf2f9a1f6a1efb497b8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-01vo-1913000000-18dc04b2a841732cef46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0119000000-c5080d81978309713b43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-3759000000-27beb72c9655a5a10292 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9641000000-e1d8ff01b979f368bc22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-237f2504c3b8b858f420 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03k9-0093000000-596171d19953284e7c67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001j-6790000000-18250cf634dd842e2d16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-b7096763c7649cee6320 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0019000000-a9bd62cc7ad7a33ce8ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9073000000-a0220d70775ae8155309 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-2aca52d377070d2c9d91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-0908000000-0cbc070d6e31e1a308b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08n9-9621000000-82835cd472af493a67f3 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-022c-9752000000-533ac31876b44d82f2aa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Intravenous, Oral, Rectal.
Rapidly absorbed following oral administration. |
|---|
| Mechanism of Toxicity | The mechanism of action of prochlorperazine has not been fully determined, but may be primarily related to its antidopaminergic effects. Prochlorperazine blocks the D2 somatodendritic autoreceptor, resulting in the blockade of postsynaptic dopamine receptors in the mesolimbic system and an increased dopamine turnover. Prochlorperazine also has anti-emetic effects, which can be attributed to dopamine blockade in the chemoreceptor trigger zone. Prochlorperazine also blocks anticholinergic and alpha-adrenergic receptors, the blockade of alpha(1)-adrenergic receptors resulting in sedation, muscle relaxation, and hypotension. |
|---|
| Metabolism | Hepatic. Undergoes metabolism in the gastric mucosa and on first pass through the liver, CYP2D6 and/or CYP3A4.
Half Life: 6 to 8 hours |
|---|
| Toxicity Values | LD50=400mg/kg (orally in mice) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the symptomatic management of psychotic disorders, short term management of nonpsychotic anxiety in patients with generalized anxiety disorder, and for the control of severe nausea and vomiting of various causes. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of central nervous system depression to the point of somnolence or coma. Agitation and restlessness may also occur. Other possible manifestations include convulsions, EKG changes and cardiac arrhythmias, fever and autonomic reactions such as hypotension, dry mouth and ileus; LD50=400mg/kg (orally in mice) |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00433 |
|---|
| HMDB ID | HMDB0014577 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | P77 |
|---|
| Wikipedia Link | Prochlorperazine |
|---|
| Chemspider ID | 4748 |
|---|
| ChEBI ID | 8435 |
|---|
| PubChem Compound ID | 4917 |
|---|
| Kegg Compound ID | C07403 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | Link |
|---|
| General References | |
|---|