| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:26:45 UTC |
|---|
| Update Date | 2016-11-09 01:08:42 UTC |
|---|
| Accession Number | CHEM002183 |
|---|
| Identification |
|---|
| Common Name | Trihexyphenidyl |
|---|
| Class | Small Molecule |
|---|
| Description | Trihexyphenidyl is only found in individuals that have used or taken this drug. It is one of the centrally acting muscarinic antagonists used for treatment of parkinsonian disorders and drug-induced extrapyramidal movement disorders and as an antispasmodic. Trihexyphenidyl is a selective M1 muscarinic acetylcholine receptor antagonist. It is able to discriminate between the M1 (cortical or neuronal) and the peripheral muscarinic subtypes (cardiac and glandular). Trihexyphenidyl partially blocks cholinergic activity in the CNS, which is responsible for the symptoms of Parkinson's disease. It is also thought to increase the availability of dopamine, a brain chemical that is critical in the initiation and smooth control of voluntary muscle movement. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
|
|---|
| Contaminant Type | - Amine
- Antidyskinetic
- Antiparkinson Agent
- Drug
- Metabolite
- Muscarinic Antagonist
- Organic Compound
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Apo-trihex | Kegg | | Trihexylphenidyl | HMDB | | Trihexylphenidyle | HMDB | | Trihexylphenizyl | HMDB | | Trihexyphenidyle | HMDB | | Triphenidyl | HMDB | | AHP brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | ApoTrihex | MeSH, HMDB | | Eisai brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Hipokinon | MeSH, HMDB | | Parkopan | MeSH, HMDB | | Trihexyphenidyl hydrochloride | MeSH, HMDB | | Trihexyphenidyl wyeth brand | MeSH, HMDB | | apo Trihex | MeSH, HMDB | | Artane | MeSH, HMDB | | Benzhexol | MeSH, HMDB | | Cypress brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Lederle brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Parkinane | MeSH, HMDB | | Psicofarma brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Rugby brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Trihexidyl hydrochloride | MeSH, HMDB | | Wyeth brand OF trihexyphenidyl | MeSH, HMDB | | Wyeth brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Apotex brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Aventis brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Schrein brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Cyclodol | MeSH, HMDB | | Hexal brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Liquipharm brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Pharmaceutical associates brand OF trihexyphenidyl hydrochloride | MeSH, HMDB | | Trihexane | MeSH, HMDB | | Trihexyphenidyl hydrochloride elixir | MeSH, HMDB |
|
|---|
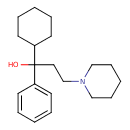
| Chemical Formula | C20H31NO |
|---|
| Average Molecular Mass | 301.466 g/mol |
|---|
| Monoisotopic Mass | 301.241 g/mol |
|---|
| CAS Registry Number | 144-11-6 |
|---|
| IUPAC Name | 1-cyclohexyl-1-phenyl-3-(piperidin-1-yl)propan-1-ol |
|---|
| Traditional Name | trihexyphenidyl |
|---|
| SMILES | OC(CCN1CCCCC1)(C1CCCCC1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C20H31NO/c22-20(18-10-4-1-5-11-18,19-12-6-2-7-13-19)14-17-21-15-8-3-9-16-21/h1,4-5,10-11,19,22H,2-3,6-9,12-17H2 |
|---|
| InChI Key | HWHLPVGTWGOCJO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Aralkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Monocyclic benzene moiety
- Piperidine
- Benzenoid
- 1,3-aminoalcohol
- Tertiary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organopnictogen compound
- Aromatic alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 258.5°C | | Boiling Point | Not Available | | Solubility | 3.14e-03 g/L |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4j-9520000000-9a2c379908fc40e03fe2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-08gm-8291000000-e91e4f7026454f2df547 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0udi-4119000000-9ed82688d3c1b39f26eb | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1190000000-eb1d6e20e193aabe8331 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-004r-9440000000-9b8665d040c0679d223d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-009i-6950000000-12b0f3e5468353070296 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-1096000000-d3df91688ef1945d673e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9130000000-5c5ffd9794999341adec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mn-9210000000-f0b1224b58d81bff09de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0019000000-7ed6535dacec03b4f64e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-9145000000-5ef99bbb769978df62bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9010000000-ee7ed12c77da3bd0134f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-3009000000-cd1bde8ffe3dde655051 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9001000000-edf90121115e0df0494d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9020000000-c9173163c6667307ea2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-2b5232fe5f3af3692a07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0319000000-5b77372872ee2d4b2758 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0390000000-a4123a20c113e31a331f | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0002-9200000000-2092a098302f5e63b76c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral.
Trihexyphenidyl is rapidly absorbed from the gastrointestinal tract. |
|---|
| Mechanism of Toxicity | Trihexyphenidyl is a selective M1 muscarinic acetylcholine receptor antagonist. It is able to discriminate between the M1 (cortical or neuronal) and the peripheral muscarinic subtypes (cardiac and glandular). Trihexyphenidyl partially blocks cholinergic activity in the CNS, which is responsible for the symptoms of Parkinson's disease. It is also thought to increase the availability of dopamine, a brain chemical that is critical in the initiation and smooth control of voluntary muscle movement. |
|---|
| Metabolism | Half Life: 3.3-4.1 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Indicated for the treatment of parkinson's disease and extrapyramidal reactions caused by drugs. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose include mydriasis, dryness of mucous membranes, red face, atonic states of bowels and bladder, and hyperthermia in high doses. Central consequences are agitation, confusion, and hallucinations. |
|---|
| Treatment | Treatment of acute overdose involves symptomatic and supportive therapy. Gastric lavage or other methods to limit absorption should be instituted. A small dose of diazepam or a short-acting barbiturate may be administered if CNS excitation is observed. Phenothiazines are contraindicated because the toxicity may be intensified due to their antimuscarinic action, causing coma. Respiratory support, artificial respiration or vasopressor agents may be necessary. Hyperpyrexia must be reversed, fluid volume replaced and acid-balance maintained. Urinary catheterization may be necessary. It is not known if Trihexyphenidyl is dialyzable. (2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 5371 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C07171 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|