| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-21 20:26:39 UTC |
|---|
| Update Date | 2016-11-09 01:08:42 UTC |
|---|
| Accession Number | CHEM002176 |
|---|
| Identification |
|---|
| Common Name | Terfenadine |
|---|
| Class | Small Molecule |
|---|
| Description | Terfenadine is only found in individuals that have used or taken this drug. In the U.S., Terfenadine was superseded by fexofenadine in the 1990s due to the risk of cardiac arrhythmia caused by QT interval prolongation.Terfenadine competes with histamine for binding at H1-receptor sites in the GI tract, uterus, large blood vessels, and bronchial muscle. This reversible binding of terfenadine to H1-receptors suppresses the formation of edema, flare, and pruritus resulting from histaminic activity. As the drug does not readily cross the blood-brain barrier, CNS depression is minimal. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Anti-Allergic Agent
- Anti-Arrhythmia Agent
- Drug
- Histamine Antagonist
- Histamine H1 Antagonist, Non-Sedating
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
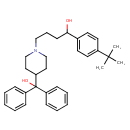
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Seldane | Kegg | | Ternadin | HMDB | | Heumann brand OF terfenadine | HMDB | | Merck dura brand OF terfenadine | HMDB | | Stadapharm brand OF terfenadine | HMDB | | Terfemundin | HMDB | | Terfenadin heumann | HMDB | | Terfenidine | HMDB | | Terfenadin von CT | HMDB | | Aliud brand OF terfenadine | HMDB | | Cantabria brand OF terfenadine | HMDB | | Mundipharma brand OF terfenadine | HMDB | | Rapidal | HMDB | | Teldane | HMDB | | Terfenadin al | HMDB | | Triludan | HMDB | | CT Arzneimittel brand OF terfenadine | HMDB | | Balkis saft spezial | HMDB | | Cyater | HMDB | | Hisfedin | HMDB | | Hoechst brand OF terfenadine | HMDB | | Terfedura | HMDB | | alpha-(4-(1,1-Dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-1-piperdinebutanol | HMDB | | CT-Arzneimittel brand OF terfenadine | HMDB | | Ratiopharm brand OF terfenadine | HMDB | | Bial brand OF terfenadine | HMDB | | Dolorgiet brand OF terfenadine | HMDB | | Sigma tau brand OF terfenadine | HMDB | | Sigma-tau brand OF terfenadine | HMDB | | Terfenadin stada | HMDB | | Terfenadin-ratiopharm | HMDB | | Wolff brand OF terfenadine | HMDB | | Terfenadin ratiopharm | MeSH |
|
|---|
| Chemical Formula | C32H41NO2 |
|---|
| Average Molecular Mass | 471.673 g/mol |
|---|
| Monoisotopic Mass | 471.314 g/mol |
|---|
| CAS Registry Number | 50679-08-8 |
|---|
| IUPAC Name | 1-(4-tert-butylphenyl)-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butan-1-ol |
|---|
| Traditional Name | terfenadine |
|---|
| SMILES | CC(C)(C)C1=CC=C(C=C1)C(O)CCCN1CCC(CC1)C(O)(C1=CC=CC=C1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3 |
|---|
| InChI Key | GUGOEEXESWIERI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Phenylbutylamine
- Phenylpropane
- Aralkylamine
- Piperidine
- Tertiary alcohol
- Tertiary aliphatic amine
- Tertiary amine
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Aromatic alcohol
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 147°C | | Boiling Point | Not Available | | Solubility | 0.0963 mg/L (at 25°C) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0922300000-f2ebf7861ae9f28463f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a4i-1091031000-8a395a9531e2fce637a4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0573-2592100000-35fee494c6fb22cf9279 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0000900000-577805b6e639de28dd59 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-0000900000-6ee8ace68773688ab606 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-000i-0000900000-8d9a4ec2424b97bb3b80 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-000i-6770900000-9830d37d285444cff791 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a4i-5920000000-909f0029205fa0a7663f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000i-2541900000-f3da0c526b0e748b9567 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0a4i-5920000000-98434dfc2057ad8cb2c6 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0a4i-5920000000-3114fcb90f8a2c560570 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000900000-6ee8ace68773688ab606 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-577805b6e639de28dd59 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-000i-6770900000-7d494f82f525a8d1b156 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-000i-0000900000-8d9a4ec2424b97bb3b80 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-000i-6770900000-9830d37d285444cff791 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0a4i-5920000000-909f0029205fa0a7663f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-000i-6770900000-3ea778438aa61069b40a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0000900000-33f5717b3bed30143d5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uki-0543900000-e5659b7fe1e1659acac9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dr-2981000000-03f1a2ca48b1a2d8e769 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0100900000-19b3063632e4afc820d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fr-5561900000-bac14174fc7e7f1ab7f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003u-9870000000-8b66dc183082f6b4a57f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-9161868ea37af548032e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0003900000-aef17866c8a1837ed79b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-2944700000-c715fedf12c50f301dff | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-001i-8890000000-2c6ad1195e498f463cbf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral.
On the basis of a mass balance study using 14C labeled terfenadine the oral absorption of terfenadine was estimated to be at least 70% |
|---|
| Mechanism of Toxicity | Terfenadine competes with histamine for binding at H1-receptor sites in the GI tract, uterus, large blood vessels, and bronchial muscle. This reversible binding of terfenadine to H1-receptors suppresses the formation of edema, flare, and pruritus resulting from histaminic activity. As the drug does not readily cross the blood-brain barrier, CNS depression is minimal. |
|---|
| Metabolism | Terfenadine is a prodrug, generally completely metabolized to the active form fexofenadine in the liver by the enzyme cytochrome P450 CYP3A4 isoform. Due to its near complete metabolism by the liver immediately after leaving the gut, terfenadine normally is not measurable in the plasma. (Wikipedia)
Half Life: 3.5 hours |
|---|
| Toxicity Values | LD50: 5000 mg/kg (Oral, mouse)
|
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of allergic rhinitis, hay fever, and allergic skin disorders. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Cardiotoxic at higher doses. In larger plasma concentrations, it may lead to toxic effects on the heart's rhythm (e.g. ventricular tachycardia and torsades de pointes). (Wikipedia) |
|---|
| Symptoms | Mild (e.g., headache, nausea, confusion), but adverse cardiac events including cardiac arrest, ventricular arrhythmias including torsades de pointes and QT prolongation have been reported. |
|---|
| Treatment | in cases of overdosage, cardiac monitoring for at least 24 hours is recommended and for as long as QTc is prolonged, along with standard measures to remove any unabsorbed drug. Treatment of the signs and symptoms of overdosage should be symptomatic and supportive after the acute stage. (2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00342 |
|---|
| HMDB ID | HMDB0014486 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Terfenadine |
|---|
| Chemspider ID | 5212 |
|---|
| ChEBI ID | 119569 |
|---|
| PubChem Compound ID | 5405 |
|---|
| Kegg Compound ID | C07463 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Timothy G. Fawcett, Christian T. Goralski, David W. Ziettlow, “Preparation of polymorphically pure terfenadine.” U.S. Patent US4742175, issued April, 1975. |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|