| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-07-03 22:19:12 UTC |
|---|
| Update Date | 2016-11-09 01:08:40 UTC |
|---|
| Accession Number | CHEM002070 |
|---|
| Identification |
|---|
| Common Name | Histrionicotoxin |
|---|
| Class | Small Molecule |
|---|
| Description | Histrionicotoxin is a toxin found in poison dart frogs (genus Dendrobates). It is a potent non-competitive antagonist of nicotinic acetylcholine receptors. (1) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | - Amine
- Animal Toxin
- Frog/Toad Toxin
- Natural Compound
- Organic Compound
|
|---|
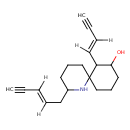
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C19H25NO |
|---|
| Average Molecular Mass | 283.415 g/mol |
|---|
| Monoisotopic Mass | 283.194 g/mol |
|---|
| CAS Registry Number | 34272-51-0 |
|---|
| IUPAC Name | 7-[(1E)-but-1-en-3-yn-1-yl]-2-[(2E)-pent-2-en-4-yn-1-yl]-1-azaspiro[5.5]undecan-8-ol |
|---|
| Traditional Name | 7-[(1E)-but-1-en-3-yn-1-yl]-2-[(2E)-pent-2-en-4-yn-1-yl]-1-azaspiro[5.5]undecan-8-ol |
|---|
| SMILES | [H]\C(CC1CCCC2(CCCC(O)C2\C([H])=C(/[H])C#C)N1)=C(\[H])C#C |
|---|
| InChI Identifier | InChI=1S/C19H25NO/c1-3-5-7-10-16-11-8-14-19(20-16)15-9-13-18(21)17(19)12-6-4-2/h1-2,5-7,12,16-18,20-21H,8-11,13-15H2/b7-5+,12-6+ |
|---|
| InChI Key | JBRYWENFVHQBGY-YTEPGLGFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as histrionicotoxins. These are frog toxins structurally characterized by the presence of a dodecahydropyrrolo[1,2-a]quinolin-1-yl}ethanol moiety or a 2,7-disubstituted 1-azaspiro[5.5]undecan-8-ol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Histrionicotoxins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Histrionicotoxins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Histrionicotoxin skeleton
- Azaspirodecane
- Piperidine
- Cyclic alcohol
- Secondary alcohol
- Acetylide
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0090000000-64706804cb43fb4f58d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0333-0390000000-92f8e5e48d998d002c98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ik9-5890000000-11b23ff54c2ee4711a33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-cc56b475fb47297af3ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-d1357f3e3dff82db9cec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02t9-0490000000-0400a22d45510fe155b0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Injection (sting/bite) (2) ; inhalation (smoking) (3) |
|---|
| Mechanism of Toxicity | Histrionicotoxin is a potent non-competitive antagonist of nicotinic acetylcholine receptors. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Histrionicotoxin is a toxin found in poison dart frogs (genus Dendrobates). (1) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Histrionicotoxin is neurotoxic. (1) |
|---|
| Symptoms | Histrionicotoxin is neurotoxic. (1) |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14134870 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|