| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-06-19 21:58:22 UTC |
|---|
| Update Date | 2016-11-09 01:08:24 UTC |
|---|
| Accession Number | CHEM000981 |
|---|
| Identification |
|---|
| Common Name | Manganese trifluoride |
|---|
| Class | Small Molecule |
|---|
| Description | Manganese trifluoride is a fluoride of manganese. Manganese is a naturally occurring metal with the symbol Mn and the atomic number 25. It does not occur naturally in its pure form, but is found in many types of rocks in combination with other substances such as oxygen, sulfur, or chlorine. Manganese occurs naturally in most foods and small amounts are needed to stay healthy, as manganese ions act as cofactors for a number of enzymes. (2, 3) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | - Fluoride Compound
- Food Toxin
- Inorganic Compound
- Manganese Compound
- Pollutant
- Synthetic Compound
|
|---|
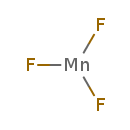
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | F3Mn |
|---|
| Average Molecular Mass | 111.933 g/mol |
|---|
| Monoisotopic Mass | 111.933 g/mol |
|---|
| CAS Registry Number | 7783-53-1 |
|---|
| IUPAC Name | trifluoromanganese |
|---|
| Traditional Name | trifluoromanganese |
|---|
| SMILES | F[Mn](F)F |
|---|
| InChI Identifier | InChI=1S/3FH.Mn/h3*1H;/q;;;+3/p-3 |
|---|
| InChI Key | SRVINXWCFNHIQZ-UHFFFAOYSA-K |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as transition metal fluorides. These are inorganic compounds in which the largest halogen atom is fluorine, and the heaviest metal atom is a transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Transition metal salts |
|---|
| Sub Class | Transition metal fluorides |
|---|
| Direct Parent | Transition metal fluorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Transition metal fluoride
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Purple/pink powder |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | > 600°C (decomp) | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-f7333413541bdba1da93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0900000000-f7333413541bdba1da93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0900000000-f7333413541bdba1da93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-12174213574b97cadfec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-12174213574b97cadfec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0900000000-12174213574b97cadfec | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (2) ; inhalation (2) |
|---|
| Mechanism of Toxicity | Manganese is a cellular toxicant that can impair transport systems, enzyme activities, and receptor functions. It primarily targets the central nervous system, particularily the globus pallidus of the basal ganglia. It is believed that the manganese ion, Mn(II), enhances the autoxidation or turnover of various intracellular catecholamines, leading to increased production of free radicals, reactive oxygen species, and other cytotoxic metabolites, along with a depletion of cellular antioxidant defense mechanisms, leading to oxidative damage and selective destruction of dopaminergic neurons. In addition to dopamine, manganese is thought to perturbations other neurotransmitters, such as GABA and glutamate. In order to produce oxidative damage, manganese must first overwhelm the antioxidant enzyme manganese superoxide dismutase. The neurotoxicity of Mn(II) has also been linked to its ability to substitute for Ca(II) under physiological conditions. It can enter mitochondria via the calcium uniporter and inhibit mitochondrial oxidative phosphorylation. It may also inhibit the efflux of Ca(II), which can result in a loss of mitochondrial membrane integrity. Mn(II) has been shown to inhibit mitochondrial aconitase activity to a significant level, altering amino acid metabolism and cellular iron homeostasis. (2) |
|---|
| Metabolism | Manganese is absorbed mainly via ingestion, but can also be inhaled. It binds to alpha-2-macroglobulin, albumin, or transferrin in the plasma and is distributed to the brain and all other mammalian tissues, though it tends to accumulate more in the liver, pancreas, and kidney. Manganese is capable of existing in a number of oxidation states and is believed to undergo changes in oxidation state within the body. Manganese oxidation state can influence tissue toxicokinetic behavior, and possibly toxicity. Manganese is excreted primarily in the faeces. (2) |
|---|
| Toxicity Values | LD50: 86 mg/kg (Oral, Rat) (4)
LC50: 320 mg/m3 (Inhalation, Rat) (4) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Chronic Inhalation: 0.0003 mg/m3 (1) |
|---|
| Health Effects | Manganese mainly affects the nervous system and may cause behavioral changes and other nervous system effects, which include movements that may become slow and clumsy. This combination of symptoms when sufficiently severe is referred to as “manganism”. (2) |
|---|
| Symptoms | Manganese mainly affects the nervous system and may cause behavioral changes and other nervous system effects, which include movements that may become slow and clumsy. This combination of symptoms when sufficiently severe is referred to as “manganism”. (2) |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Manganese(III) fluoride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 82213 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|