| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-06-17 23:53:02 UTC |
|---|
| Update Date | 2016-11-09 01:08:22 UTC |
|---|
| Accession Number | CHEM000813 |
|---|
| Identification |
|---|
| Common Name | Cycloate |
|---|
| Class | Small Molecule |
|---|
| Description | Cycloate is selective thiocarbamate herbicide which will provide effective preemergence control of nutsedge (Cyperus spp.) and annual grasses. Broadleaf weeds such as black nightshade (Solanum nigrum), hairy nightshade (Solanum villosum), henbit (Lamium spp.), lambsquarters (Chenopodium album), purslane (Portulaca oleracea), redroot pigweed (Amaranthus retroflexus), shepherdspurse (Capsella bursapastoris), and small stinging nettle (burning nettle) can also be controlled with this herbicide. Thiocarbamates are mainly used in agriculture as insecticides, herbicides, and fungicides. Additional uses are as biocides for industrial or other commercial applications, and in household products. Some are used for vector control in public health. Thiocarbamates are mostly liquids or solids with low melting points.
|
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- HPV EPA Chemicals
- My Exposome Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Amine
- Carbamate
- Ether
- Herbicide
- Organic Compound
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cycloic acid | Generator | | Ro-neet | MeSH | | Roneet | MeSH | | S-Ethyl-N-cyclohexylthiocarbamate | MeSH | | Cycleate | MeSH | | Ethsane | MeSH | | Etsan | MeSH |
|
|---|
| Chemical Formula | C11H21NOS |
|---|
| Average Molecular Mass | 215.356 g/mol |
|---|
| Monoisotopic Mass | 215.134 g/mol |
|---|
| CAS Registry Number | 1134-23-2 |
|---|
| IUPAC Name | N-cyclohexyl-N-ethyl(ethylsulfanyl)formamide |
|---|
| Traditional Name | cycloate |
|---|
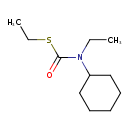
| SMILES | CCSC(=O)N(CC)C1CCCCC1 |
|---|
| InChI Identifier | InChI=1S/C11H21NOS/c1-3-12(11(13)14-4-2)10-8-6-5-7-9-10/h10H,3-9H2,1-2H3 |
|---|
| InChI Key | DFCAFRGABIXSDS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiocarbamic acid derivatives. These are organic compounds containing a functional group with the general structure OC(=S)NR2 or SC(=O)NR2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Thiocarbonyl compounds |
|---|
| Sub Class | Thiocarbamic acid derivatives |
|---|
| Direct Parent | Thiocarbamic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiocarbamic acid derivative
- Carbonic acid derivative
- Sulfenyl compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 11.5°C | | Boiling Point | Not Available | | Solubility | 0.085 mg/mL at 22°C [SHIU,WY et al. (1990)] |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003r-9600000000-8e2fb40b722933a7889f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-5490000000-aef849d1f9655a9a97e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w4i-7920000000-8363e613eff2215f23c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-9100000000-51941b4a270e331dd96f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-4970000000-b36b721a030b6a288c19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0imi-5910000000-61c35786dd68ccd5e90e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-5900000000-07a29c8bbb3fac41dce0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-9440000000-3bcba50c495a72549bdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9200000000-fec3d450c05cdf8d838f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9100000000-ea3fde4fe9b617c53ab3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-7190000000-bf88e1da2506eb7a0469 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-749fb73a3614fe71646e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-9000000000-6a7d6aa09f04a77e5e7e | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0kcr-9200000000-0c5f2692d5afc5826868 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (1) ; oral (1); dermal (1) |
|---|
| Mechanism of Toxicity | Some thiocarbamates (EPTC, Molinate, Pebulate, and Cycloate) share a common mechanism of toxicity, i.e. the inhibition of acetylcholinesterase. An acetylcholinesterase inhibitor suppresses the action of acetylcholine esterase. Because of its essential function, chemicals that interfere with the action of acetylcholine esterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses. Headache, salivation, nausea, vomiting, abdominal pain and diarrhea are often prominent at higher levels of exposure. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. |
|---|
| Metabolism | As a general rule, thiocarbamates can be absorbed via the skin, mucous membranes, and the respiratory and gastrointestinal tracts. They are eliminated quite rapidly, mainly via expired air and urine. Two major pathways exist for the metabolism of thiocarbamates in mammals. One is via sulfoxidation and conjugation with glutathione. The conjugation product is then cleaved to a cysteine derivative, which is metabolized to a mercapturic acid compound. The second route is oxidation of the sulfur to a sulfoxide, which is then oxidized to a sulfone, or hydroxylation to compounds that enter the carbon metabolic pool. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Thiocarbamates are widely used throughout the world and are produced in great quantities, mainly as herbicides and fungicides. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Data concerning the effects of thiocarbamates on man are scarce. However, cases of irritation and sensitization have been observed among agricultural workers. Some thiocarbamates, e.g., molinate, have an effect on sperm morphology and, consequently, on reproduction. However, no teratogenic effects have been observed. The results of mutagenicity studies have shown that thiocarbamates containing dichloroallyl groups are highly mutagenic. Some thiocarbamates are acetylcholine esterase inhibitors. Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. |
|---|
| Symptoms | As with organophosphates, the signs and symptoms are based on excessive cholinergic stimulation. Unlike organophosphate poisoning, carbamate poisonings tend to be of shorter duration because the inhibition of nervous tissue acetylcholinesterase is reversible, and carbamates are more rapidly metabolized. Muscle weakness, dizziness, sweating and slight body discomfort are commonly reported early symptoms. Headache, salivation, nausea, vomiting, abdominal pain and diarrhea are often prominent at higher levels of exposure. Contraction of the pupils with blurred vision, incoordination, muscle twitching and slurred speech have been reported. (2) |
|---|
| Treatment | Treatment of carbamate poisoning is similar to that of organophosphate poisoning in that atropine sulfate injections readily reverse the effects. For acute exposures and first aid: EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0250636 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 13698 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14337 |
|---|
| Kegg Compound ID | C18780 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|