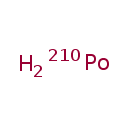| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-03-06 18:58:07 UTC |
|---|
| Update Date | 2016-11-09 01:08:10 UTC |
|---|
| Accession Number | CHEM000112 |
|---|
| Identification |
|---|
| Common Name | Polonium-210 |
|---|
| Class | Small Molecule |
|---|
| Description | Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie and Pierre Curie. A rare and highly radioactive metalloid, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. When it is mixed or alloyed with beryllium, polonium can be a neutron source and has been used in this capacity as a neutron trigger or initiator for nuclear weapons. Polonium has also been studied for possible use in heating spacecraft. It is unstable and all isotopes of polonium are radioactive. It is one ingredient of cigarette. (2) |
|---|
| Contaminant Sources | - IARC Carcinogens Group 1
- T3DB toxins
- Tobacco Smoke Compounds
|
|---|
| Contaminant Type | - Cigarette Toxin
- Inorganic Compound
- Metal
- Metalloid
- Natural Compound
- Plutonium Compound
- Pollutant
- Radioactive
- Radioactive Isotope
|
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | H2Po |
|---|
| Average Molecular Mass | 211.999 g/mol |
|---|
| Monoisotopic Mass | 211.999 g/mol |
|---|
| CAS Registry Number | 13981-52-7 |
|---|
| IUPAC Name | (²¹⁰Po)polane |
|---|
| Traditional Name | (²¹⁰Po)polane |
|---|
| SMILES | [210PoH2] |
|---|
| InChI Identifier | InChI=1S/Po.2H/i1+1;; |
|---|
| InChI Key | OFSDTGZOZPQDCK-GOCMCNPZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as miscellaneous mixed metal/non-metals. These are inorganic compounds containing non-metal as well as metal atoms but not belonging to afore mentioned classes. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Miscellaneous mixed metal/non-metals |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Miscellaneous mixed metal/non-metals |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | - Miscellaneous mixed metal/non-metal
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-70316c48f27e95f2905b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-70316c48f27e95f2905b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0090000000-70316c48f27e95f2905b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-7364005eed22e2c1054a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0090000000-7364005eed22e2c1054a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0090000000-7364005eed22e2c1054a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (2); Inhalation (2) |
|---|
| Mechanism of Toxicity | The alpha radiation polonium emits does not penetrate the skin but can irradiate internal organs when polonium is inhaled or ingested. The ionizing radiation produced by plutonium causes cellular damage that includes DNA breakage, accurate or inaccurate repair, apoptosis, gene mutations, chromosomal change, and genetic instability. This leads to loss of normal cell and tissue homeostasis, and development of malignancy. Ionizing radiation that does not directly damage DNA can produce reactive oxygen intermediates that directly affect the stability of p53, an important enzyme in cell-cycle regulation, and produce oxidative damage to individual bases in DNA and point mutations by mispairing during DNA replication. (1, 2) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (4) |
|---|
| Uses/Sources | When it is mixed or alloyed with beryllium, polonium can be a neutron source and has been used in this capacity as a neutron trigger or initiator for nuclear weapons. Polonium has also been studied for possible use in heating spacecraft. (2) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Polonium's radioactivity can cause cancer, especially of the lung, if ingested of inhaled. (2) |
|---|
| Symptoms | Exposure to high doses of ionizing radiation results in acute radiation syndrome, which can cause skin burns, hair loss, nausea, vomiting, dizziness, disorientation, low blood pressure, headache, fatigue, weakness, fever, birth defects, illness, infection, and death. (1, 3) |
|---|
| Treatment | Chelation agents such as British Anti-Lewisite (dimercaprol) can be used to decontaminate humans. Treatment reversing the effects of irradiation is currently not possible. Anaesthetics and antiemetics are administered to counter the symptoms of exposure, as well as antibiotics for countering secondary infections due to the resulting immune system deficiency. (3, 2) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 61710 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|