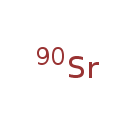| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2009-03-06 18:58:07 UTC |
|---|
| Update Date | 2016-11-09 01:08:10 UTC |
|---|
| Accession Number | CHEM000110 |
|---|
| Identification |
|---|
| Common Name | Strontium-90 |
|---|
| Class | Small Molecule |
|---|
| Description | Strontium is a naturally occurring element found in rocks, soil, dust, coal, and oil. Strontium-90 is a radioactive isotope of strontium (Sr) with a half-life of 28.8 years. Strontium-90 is a product of nuclear fission and is obtained during nuclear reprocessing of spent nuclear fuel and is a beta-emitter. Strontium 90 is extensively used in medicine and industry. Strontium-90 is a radioactivity hazard and is linked to cancers. (2, 4) |
|---|
| Contaminant Sources | - IARC Carcinogens General
- IARC Carcinogens Group 1
- T3DB toxins
|
|---|
| Contaminant Type | - Industrial/Workplace Toxin
- Inorganic Compound
- Metal
- Natural Compound
- Pollutant
- Radioactive
- Radioactive Isotope
- Strontium Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 90Sr Radioisotope | MeSH | | SR-90 Radioisotope | MeSH | | Radioisotopes, strontium | MeSH | | Strontium radioisotopes | MeSH |
|
|---|
| Chemical Formula | Sr |
|---|
| Average Molecular Mass | 89.908 g/mol |
|---|
| Monoisotopic Mass | 89.908 g/mol |
|---|
| CAS Registry Number | 10098-97-2 |
|---|
| IUPAC Name | (⁹⁰Sr)strontium |
|---|
| Traditional Name | (⁹⁰Sr)strontium |
|---|
| SMILES | [90Sr] |
|---|
| InChI Identifier | InChI=1S/Sr/i1+2 |
|---|
| InChI Key | CIOAGBVUUVVLOB-NJFSPNSNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as homogeneous alkaline earth metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a alkaline earth metal atom. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous metal compounds |
|---|
| Class | Homogeneous alkaline earth metal compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Homogeneous alkaline earth metal compounds |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | - Homogeneous alkaline earth metal
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9000000000-bbbb0a7145915cdb57ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-bbbb0a7145915cdb57ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-bbbb0a7145915cdb57ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-1a62eef0a27c7459c31a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-1a62eef0a27c7459c31a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9000000000-1a62eef0a27c7459c31a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (2) |
|---|
| Mechanism of Toxicity | The fact that strontium is chemically similar to calcium allows it to exchange for calcium in bone. It affects bone development and strength by binding directly to hydroxyapatite crystals, which interferes with the normal crystalline structure of bone. Strontium can also interact with secondary cell messenger systems and transporter systems that normally use calcium. It is thought to bind to the calcium receptor of the parathyroid gland, thereby suppressing parathyroid hormone levels, preventing vitamin D3 activiation, and reducing calcium absorption. The ionizing radiation produced by strontium-90 causes cellular damage that includes DNA breakage, accurate or inaccurate repair, apoptosis, gene mutations, chromosomal change, and genetic instability. This leads to loss of normal cell and tissue homeostasis, and development of malignancy. Strontium's ability to mimick calcium makes bone cancer a particular risk. Ionizing radiation that does not directly damage DNA can produce reactive oxygen intermediates that directly affect the stability of p53, an important enzyme in cell-cycle regulation, and produce oxidative damage to individual bases in DNA and point mutations by mispairing during DNA replication. (3, 4) |
|---|
| Metabolism | The radioactivity of strontium-90 allow it to penetrate the body following inhalation, ingestion, or dermal exposure. Strontium-90 exhibits biochemical behavior similar to calcium. After entering the organism, about 70-80% of the dose is excreted. Virtually all remaining strontium-90 is deposited in bones and bone marrow, with the remaining 1% remaining in blood and soft tissues. The metabolism of strontium consists of binding interactions with proteins and, based on its similarity to calcium, probably complex formation with various inorganic anions such as carbonate and phosphate, and carboxylic acids such as citrate and lactate. Strontium-90 is eliminated mainly in the urine and faeces. (2) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (6) |
|---|
| Uses/Sources | Strontium-90 is widely used in medicine and industry, as a radioactive source for thickness gauges, and for superficial radiotherapy of some cancers. Being cheaper than the alternative 238Pu, it is used as a heat source in many Russian and Soviet radioisotope thermoelectric generators. It is also used as a radioactive tracer in medicine and agriculture. (2) |
|---|
| Minimum Risk Level | Intermediate Oral: 2 mg/kg/day (1) |
|---|
| Health Effects | High levels of radioactive strontium can damage bone marrow, cause anemia and prevent the blood from clotting properly. Strontium-90 present in bones could cause bone cancer, cancer of nearby tissues, and leukemia. (2, 4) |
|---|
| Symptoms | High levels of radioactive strontium can damage bone marrow, cause anemia and prevent the blood from clotting properly. Exposure to high doses of ionizing radiation results in acute radiation syndrome, which can cause skin burns, hair loss, nausea, vomiting, dizziness, disorientation, low blood pressure, headache, fatigue, weakness, fever, birth defects, illness, infection, and death. (3, 5, 4) |
|---|
| Treatment | Treatment reversing the effects of irradiation is currently not possible. Anaesthetics and antiemetics are administered to counter the symptoms of exposure, as well as antibiotics for countering secondary infections due to the resulting immune system deficiency. (5) |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Strontium-90 |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5486204 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|