| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-06-03 11:20:28 UTC |
|---|
| Update Date | 2016-11-09 01:23:00 UTC |
|---|
| Accession Number | CHEM043846 |
|---|
| Identification |
|---|
| Common Name | Propanoic acid, 2-[4-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenoxy]-, (2R)- |
|---|
| Class | Small Molecule |
|---|
| Description | A 2-(4-{oxy}phenoxy)propanoic acid that has R configuration. It is the active enantiomer of the herbicide fluazifop and is the major metabolite of fluazifop-P-butyl. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
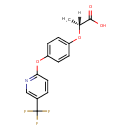
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-(R)-Fluazifop | ChEBI | | (+)-Fluazifop | ChEBI | | (2R)-2-[4-[[5-(Trifluoromethyl)-2-pyridinyl]oxy]phenoxy]propanoic acid | ChEBI | | (R)-(+)-Fluazifop | ChEBI | | (R)-2-{4-[5-(trifluoromethyl)-2-pyridyloxy]phenoxy}propionic acid | ChEBI | | (R)-Fluazifop | ChEBI | | (2R)-2-[4-[[5-(Trifluoromethyl)-2-pyridinyl]oxy]phenoxy]propanoate | Generator | | (R)-2-{4-[5-(trifluoromethyl)-2-pyridyloxy]phenoxy}propionate | Generator | | (2R)-2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoate | Generator |
|
|---|
| Chemical Formula | C15H12F3NO4 |
|---|
| Average Molecular Mass | 327.259 g/mol |
|---|
| Monoisotopic Mass | 327.072 g/mol |
|---|
| CAS Registry Number | 83066-88-0 |
|---|
| IUPAC Name | (2R)-2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid |
|---|
| Traditional Name | (2R)-2-(4-{[5-(trifluoromethyl)pyridin-2-yl]oxy}phenoxy)propanoic acid |
|---|
| SMILES | [H][C@](C)(OC1=CC=C(OC2=NC=C(C=C2)C(F)(F)F)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H12F3NO4/c1-9(14(20)21)22-11-3-5-12(6-4-11)23-13-7-2-10(8-19-13)15(16,17)18/h2-9H,1H3,(H,20,21)/t9-/m1/s1 |
|---|
| InChI Key | YUVKUEAFAVKILW-SECBINFHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryloxyphenoxypropionic acids. These are aromatic compounds containing a phenoxypropionic acid that is para-substituted with an aryl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | 2-phenoxypropionic acids |
|---|
| Direct Parent | Aryloxyphenoxypropionic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aryloxyphenoxypropionic acid
- Diaryl ether
- Phenoxyacetate
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Pyridine
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Alkyl halide
- Organic oxygen compound
- Alkyl fluoride
- Organooxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | - 2-(4-\{[5-(trifluoromethyl)pyridin-2-yl]oxy\}phenoxy)propanoic acid (CHEBI:83599 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0019000000-de09c04c70f8863bb629 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufr-0197000000-17d2cadf02a64746ad14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-0910000000-0f2ba3e939bd2d6d3d04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0019000000-7115c0045d1b001c824c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3293000000-df265b05519b9f45b14b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9470000000-cc8ae86bc74bedec9d98 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 83599 |
|---|
| PubChem Compound ID | 91733 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. | | 2. | | 3. | | 4. | | 5. | | 6. | | 7. | | 8. | | 9. |
|
|---|