| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 23:19:23 UTC |
|---|
| Update Date | 2016-11-09 01:18:21 UTC |
|---|
| Accession Number | CHEM026917 |
|---|
| Identification |
|---|
| Common Name | 2,6-Dimethyl-1,4-benzenediol |
|---|
| Class | Small Molecule |
|---|
| Description | 2,6-Dimethyl-1,4-benzenediol is found in common pea. Claimed isolation from Pisum sativum (pea) |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
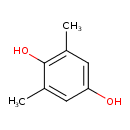
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2, 6-Dimethyl-P-benzohydroquinone | HMDB | | 2, 6-Xylohydroquinone | HMDB | | 2,5-Dihydroxy-m-xylene | HMDB | | 2,6-Dimethyl-hydroquinone | HMDB | | 2,6-Dimethyl-P-benzohydroquinone | HMDB | | 2,6-Dimethylbenzene-1,4-diol | HMDB | | 2,6-Dimethylhydroquinone | HMDB | | 2,6-Dimethylhydroquinone, 8ci | HMDB | | 2,6-Dimethylquinol | HMDB | | 2,6-Xylohydroquinone | HMDB | | 2,6-Xyloquinol | HMDB | | 3,5-Dimethylhydroquinone | HMDB | | DMHQ | HMDB | | m-XHQ | HMDB | | m-Xylene-2,5-diol | HMDB | | m-Xylohydroquinone | HMDB | | Metaxylohydroquinone | HMDB | | MXHQ | HMDB | | Poly(2,6-dimethyl-1,4-phenylene oxide) | HMDB |
|
|---|
| Chemical Formula | C8H10O2 |
|---|
| Average Molecular Mass | 138.164 g/mol |
|---|
| Monoisotopic Mass | 138.068 g/mol |
|---|
| CAS Registry Number | 654-42-2 |
|---|
| IUPAC Name | 2,6-dimethylbenzene-1,4-diol |
|---|
| Traditional Name | 1,4-benzenediol, 2,6-dimethyl- |
|---|
| SMILES | CC1=CC(O)=CC(C)=C1O |
|---|
| InChI Identifier | InChI=1S/C8H10O2/c1-5-3-7(9)4-6(2)8(5)10/h3-4,9-10H,1-2H3 |
|---|
| InChI Key | SGWZVZZVXOJRAQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroquinones. Hydroquinones are compounds containing a hydroquinone moiety, which consists of a benzene ring with a hydroxyl groups at positions 1 and 4. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenediols |
|---|
| Direct Parent | Hydroquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - M-xylene
- Xylene
- O-cresol
- M-cresol
- Hydroquinone
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-1900000000-88ff73c8d1623ee40a29 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-5490000000-f8ef259a1d967e78dd1c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-e4a9e1743830760fbb61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-dc1c84c5524e01153f43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9600000000-beafda0b0f54b42a956b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-08ef9d705ca98ce39abf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1900000000-2f78f289353ccbdd5534 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9300000000-09309f2133472f16330f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-b1389ce8d1190e7088d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1900000000-8d53d61708dd20fdb467 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0076-9300000000-13a8e5bb83e69dab8b90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-a988d5277867358ca91a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-9800000000-9e041e506c5c739100ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6x-9000000000-bdfa6ce17abc04785c51 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-000i-9700000000-540233787be2454eb216 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032137 |
|---|
| FooDB ID | FDB008863 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00058169 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 62764 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 69560 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|