| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 22:26:27 UTC |
|---|
| Update Date | 2016-11-09 01:18:06 UTC |
|---|
| Accession Number | CHEM025511 |
|---|
| Identification |
|---|
| Common Name | Trideca-1,3,5-trien-7,9,11-triyn |
|---|
| Class | Small Molecule |
|---|
| Description | (3E,5Z)-1,3,5-Tridecatriene-7,9,11-triyne is isolated from Artemisia vulgaris (mugwort). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
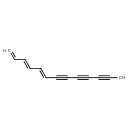
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C13H10 |
|---|
| Average Molecular Mass | 166.219 g/mol |
|---|
| Monoisotopic Mass | 166.078 g/mol |
|---|
| CAS Registry Number | 126381-91-7 |
|---|
| IUPAC Name | (3E,5E)-trideca-1,3,5-trien-7,9,11-triyne |
|---|
| Traditional Name | (3E,5E)-trideca-1,3,5-trien-7,9,11-triyne |
|---|
| SMILES | CC#CC#CC#C\C=C\C=C\C=C |
|---|
| InChI Identifier | InChI=1S/C13H10/c1-3-5-7-9-11-13-12-10-8-6-4-2/h3,5,7,9,11H,1H2,2H3/b7-5+,11-9+ |
|---|
| InChI Key | AJWRNFIZKHPOHC-JEGFTUTRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as enynes. These are hydrocarbons containing an alkene and an alkyne group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Enynes |
|---|
| Direct Parent | Enynes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Enyne
- Unsaturated aliphatic hydrocarbon
- Olefin
- Acyclic olefin
- Acyclic acetylene
- Acetylene
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-5900000000-0ee64df4c6b05966e5d4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1900000000-b438c29f7769f22f9152 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-5900000000-3adabf34ff05ba1f12f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-9200000000-17650d5d688708d7d4d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-2266a765b43371823aad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-d764ff14d0c9a08fe78b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-9800000000-3aacac7259dd69c874c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014l-9800000000-fb049488771ccac04251 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002o-9100000000-f6725d752e274b50ddda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9100000000-f6cc5b88b37c3f0d4a77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-efb20e965011221811a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-0b916ad49a7bba0d083d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-5900000000-6d1ff08c0f3431f2459a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039104 |
|---|
| FooDB ID | FDB004173 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4479622 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5322029 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|