| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 03:16:55 UTC |
|---|
| Update Date | 2016-11-09 01:17:18 UTC |
|---|
| Accession Number | CHEM021232 |
|---|
| Identification |
|---|
| Common Name | Norcotinine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
- Suspected Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
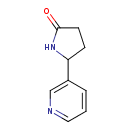
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (RS)-Norcotinine | HMDB | | 5-(3-Pyridinyl)-2-pyrrolidinone | HMDB | | Demethylcotinine | HMDB | | Norcotinine, (+-)-isomer | MeSH, HMDB | | Norcotinine, (R)-isomer | MeSH, HMDB | | Norcotinine, (S)-isomer | MeSH, HMDB |
|
|---|
| Chemical Formula | C9H10N2O |
|---|
| Average Molecular Mass | 162.189 g/mol |
|---|
| Monoisotopic Mass | 162.079 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-(pyridin-3-yl)pyrrolidin-2-one |
|---|
| Traditional Name | 5-pyridin-3-ylpyrrolidin-2-one |
|---|
| SMILES | O=C1CCC(N1)C1=CN=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H10N2O/c12-9-4-3-8(11-9)7-2-1-5-10-6-7/h1-2,5-6,8H,3-4H2,(H,11,12) |
|---|
| InChI Key | FXFANIORDKRCCA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridines and derivatives. Pyridines and derivatives are compounds containing a pyridine ring, which is a six-member aromatic heterocycle which consists of one nitrogen atom and five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pyridines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridine
- Heteroaromatic compound
- Cyclic carboximidic acid
- Pyrroline
- Lactim
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06rx-6900000000-7167061e9fe9558a0456 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-6b212cf317cd02f3fb27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-1900000000-47e1891277a98c78b7d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v0u-9400000000-a2be02f0fbf088451120 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-f03246b316270169b004 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1900000000-9816a6273230e64c8a15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-94d564ccce13bc3f2793 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-68b80ae98d7436c26dac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-3900000000-dc0d7910e90353c87b8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9300000000-c6cdc7817919d9ca67b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-d99f4e50b73cef46d299 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1900000000-2a1c38a43f8c2aa32179 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00mo-9100000000-60ef78843c02c161575c | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001297 |
|---|
| FooDB ID | FDB022540 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6142 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 400 |
|---|
| ChEBI ID | 89406 |
|---|
| PubChem Compound ID | 413 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Dewey, Lovell J. Metabolites of nicotine and a synthesis of nornicotine. Journal of the American Chemical Society (1958), 80 1634-6. | | 2. Zuccaro P, Altieri I, Rosa M, Passa AR, Pichini S, Ricciarello G, Pacifici R: Determination of nicotine and four metabolites in the serum of smokers by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1993 Nov 24;621(2):257-61. | | 3. Kyerematen GA, Morgan ML, Chattopadhyay B, deBethizy JD, Vesell ES: Disposition of nicotine and eight metabolites in smokers and nonsmokers: identification in smokers of two metabolites that are longer lived than cotinine. Clin Pharmacol Ther. 1990 Dec;48(6):641-51. | | 4. McManus KT, deBethizy JD, Garteiz DA, Kyerematen GA, Vesell ES: A new quantitative thermospray LC-MS method for nicotine and its metabolites in biological fluids. J Chromatogr Sci. 1990 Oct;28(10):510-6. | | 5. Kim I, Huestis MA: A validated method for the determination of nicotine, cotinine, trans-3'-hydroxycotinine, and norcotinine in human plasma using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. J Mass Spectrom. 2006 Jun;41(6):815-21. |
|
|---|