| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:05:43 UTC |
|---|
| Update Date | 2016-11-09 01:15:33 UTC |
|---|
| Accession Number | CHEM017247 |
|---|
| Identification |
|---|
| Common Name | 2,6-Dimethyl-2,4,6-octatriene |
|---|
| Class | Small Molecule |
|---|
| Description | 2,6-dimethyl-2,4,6-octatriene, also known as alloocimene, (e,z)-isomer or allo-ocimene, is a member of the class of compounds known as acyclic monoterpenoids. Acyclic monoterpenoids are monoterpenes that do not contain a cycle. 2,6-dimethyl-2,4,6-octatriene can be found in parsnip, sweet basil, and tarragon, which makes 2,6-dimethyl-2,4,6-octatriene a potential biomarker for the consumption of these food products. 2,6-dimethyl-2,4,6-octatriene can be found primarily in saliva. |
|---|
| Contaminant Sources | - FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
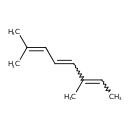
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C10H16 |
|---|
| Average Molecular Mass | 136.234 g/mol |
|---|
| Monoisotopic Mass | 136.125 g/mol |
|---|
| CAS Registry Number | 673-84-7 |
|---|
| IUPAC Name | 2,6-dimethylocta-2,4,6-triene |
|---|
| Traditional Name | 2,6-dimethyl-octa-2,4,6-triene |
|---|
| SMILES | CC=C(C)C=CC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C10H16/c1-5-10(4)8-6-7-9(2)3/h5-8H,1-4H3 |
|---|
| InChI Key | GQVMHMFBVWSSPF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic monoterpenoids. These are monoterpenes that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Acyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic monoterpenoid
- Branched unsaturated hydrocarbon
- Alkatriene
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Acyclic olefin
- Hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05i3-9300000000-6803c5aec9b747123229 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-3900000000-568bf0e6523964538a2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-9400000000-95598295eb99b04c65be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxr-9000000000-590049ddc8eb5a35bf5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-a4739c0d05c12a3bc3ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1900000000-78a25bb797b370a39f8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9600000000-9928cbbb5fa1200f4f55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-efb2ccdf7ec684be7ec0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-d0df8bba9aea5d4ee599 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-749e71535a33d75a6938 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05o0-9200000000-45090ca10e8536a84b92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-9000000000-cf1269fdbb188d6bf5a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v00-9000000000-b77b4645d9ce6c95a53d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0061789 |
|---|
| FooDB ID | FDB006262 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 12137 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5368821 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|