| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 03:45:59 UTC |
|---|
| Update Date | 2016-11-09 01:14:27 UTC |
|---|
| Accession Number | CHEM011857 |
|---|
| Identification |
|---|
| Common Name | Dithianon |
|---|
| Class | Small Molecule |
|---|
| Description | A naphthodithiin that is 5,10-dioxo-5,10-dihydronaphtho[2,3-b][1,4]dithiin which is substituted by nitrile groups at positions 2 and 3. It is a broad spectrum fungicide used to control scab, downy mildew, rust, and leaf spot in the commercial growing of grapes and other fruit, citrus, coffee, and vegetables. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HPV EPA Chemicals
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
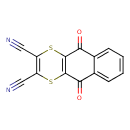
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-Dithiaanthraquinone-2,3-dinitrile | ChEBI | | 2,3-Dicarbonitrilo-1,4-diathiaanthrachinon | ChEBI | | 2,3-Dicyano-1,4-dithia-anthraquinone | ChEBI | | 2,3-Dinitrilo-1,4-dithia-9,10-anthraquinone | ChEBI | | 2,3-Dinitrilo-1,4-dithia-anthraquinone | ChEBI | | 5,10-Dihydro-5,10-dioxonaphtho[2,3-b]-1,4-dithiin-2,3-dicarbonitrile | ChEBI | | Delan | ChEBI | | Dithianone | ChEBI | | 1, 4-Dithiaanthraquinone-2,3-dinitrile | HMDB | | 1,4-Dithiaanthraquinone-2,3-dicarbonitrile | HMDB | | 2,3-Dicyano-1, 4-dithiaanthraquinone | HMDB | | 2,3-Dicyano-1,4-dithiaanthraquinone | HMDB | | 2,3-Dinitrilo-1,4-dithiaanthraquinone | HMDB | | 2,3-Dinitrilo-1,4-dithioanthrachinon | HMDB | | 5,10-Dihydro-5,10-dioxonaphtho[2,3-b]-1,4-dithiin-2,3-dicarbonitrile, 9ci | HMDB | | Delan (fungicide) | HMDB | | Delan WP | HMDB | | Delan-col | HMDB | | DTA | HMDB | | Merkdelan | HMDB | | Stauffer MV-119a | HMDB | | Thynon | HMDB |
|
|---|
| Chemical Formula | C14H4N2O2S2 |
|---|
| Average Molecular Mass | 296.324 g/mol |
|---|
| Monoisotopic Mass | 295.971 g/mol |
|---|
| CAS Registry Number | 3347-22-6 |
|---|
| IUPAC Name | 5,10-dioxo-5H,10H-naphtho[2,3-b][1,4]dithiine-2,3-dicarbonitrile |
|---|
| Traditional Name | delan |
|---|
| SMILES | O=C1C2=C(SC(C#N)=C(S2)C#N)C(=O)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C14H4N2O2S2/c15-5-9-10(6-16)20-14-12(18)8-4-2-1-3-7(8)11(17)13(14)19-9/h1-4H |
|---|
| InChI Key | PYZSVQVRHDXQSL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthalenes. Naphthalenes are compounds containing a naphthalene moiety, which consists of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthalene
- Benzodithiin
- 1,4-benzodithiin
- Heteroaromatic compound
- Organoheterocyclic compound
- Nitrile
- Carbonitrile
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-2190000000-8a7b1b7ccc6daf025528 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-254ad508ab6bf768be5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-254ad508ab6bf768be5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9020000000-15e81b644b367e6262e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-96f892822cb96918d93c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-a191f8792b4866791ead | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0090000000-0e8e892f8affebd6ccbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-fd729b45939bfa2a1f35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-fd729b45939bfa2a1f35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052e-0790000000-458fc67a3226f2b421a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-764fef44dbeab67a1c93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-764fef44dbeab67a1c93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0090000000-764fef44dbeab67a1c93 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031780 |
|---|
| FooDB ID | FDB008453 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17724 |
|---|
| ChEBI ID | 81842 |
|---|
| PubChem Compound ID | 18771 |
|---|
| Kegg Compound ID | C18574 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|