| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:16:36 UTC |
|---|
| Update Date | 2016-11-09 01:21:26 UTC |
|---|
| Accession Number | CHEM036160 |
|---|
| Identification |
|---|
| Common Name | Sialyllacto-N-neotetraose c |
|---|
| Class | Small Molecule |
|---|
| Description | Sialyllacto-N-neotetraose c is an oligosaccharide found in human breast milk. Two additional sialylated pentasaccharides include sialyllacto-N-tetraose-a and -b. Oligosaccharides containing N-acetyl (or N-glycolyl) neuraminic acid, i.e. sialylated oligosaccharides, are important components of glycoproteins and glycolipids. In nature, sialylated oligosaccharides often occur as homologous series, with incremental differences in composition, and as structural isomers with subtle differences in monosaccharide sequence and glycosyl linkage and, possibly, with presence or absence of molecular branching. A large number of such sialylated oligosaccharides occur in human milk, where they may have important biological functions. Major structural isomers of these acidic oligosaccharides in human milk are 3'- and 6'-sialyllactoses and the sialyllacto-N-tetraoses; appreciable amounts of 3'- and 6'-sialyllactosamines are found in human urine. (PMID:11471815). Oligosaccharides in human milk inhibit enteric pathogens in vitro and in vivo. Neutral milk oligosaccharides vary among individuals and over the course of lactation. (PMID:10683228). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
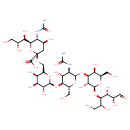
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LS-Tetrasaccharide c | HMDB | | LSTC | HMDB | | O-(N-Acetyl-alpha-neuraminosyl)-(2->6)-O-beta-D-galactopyranosyl-(1->4)-O-2-(acetylamino)-2-deoxy-beta-D-glucopyranosyl-(1->3)-O-beta-D-galactopyranosyl-(1->4)-D-glucose | HMDB | | O-(N-Acetyl-alpha-neuraminosyl)-(2->6)-O-beta-delta-galactopyranosyl-(1->4)-O-2-(acetylamino)-2-deoxy-beta-delta-glucopyranosyl-(1->3)-O-beta-delta-galactopyranosyl-(1->4)-delta-glucose | HMDB | | SLNT-c | HMDB | | (2R,4S,5R,6R)-2-{[(3R,4S,5R,6S)-6-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | Generator, HMDB |
|
|---|
| Chemical Formula | C37H62N2O29 |
|---|
| Average Molecular Mass | 998.884 g/mol |
|---|
| Monoisotopic Mass | 998.344 g/mol |
|---|
| CAS Registry Number | 64003-55-0 |
|---|
| IUPAC Name | (2R,4S,5R,6R)-2-{[(3R,4S,5R,6S)-6-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-5-acetamido-4-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2R,4S,5R,6R)-2-{[(3R,4S,5R,6S)-6-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-5-acetamido-4-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| SMILES | CC(=O)N[C@@H]1[C@@H](O)[C@H](O[C@@H]2OC(CO[C@@]3(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O3)[C@H](O)[C@H](O)CO)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)[C@@H](CO)O[C@H]1O[C@H]1[C@@H](O)[C@@H](CO)O[C@@H](O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)C=O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C37H62N2O29/c1-10(45)38-19-12(47)3-37(36(59)60,68-31(19)22(52)14(49)5-41)61-9-18-23(53)26(56)27(57)34(64-18)66-30-17(8-44)63-33(20(25(30)55)39-11(2)46)67-32-24(54)16(7-43)62-35(28(32)58)65-29(15(50)6-42)21(51)13(48)4-40/h4,12-35,41-44,47-58H,3,5-9H2,1-2H3,(H,38,45)(H,39,46)(H,59,60)/t12-,13-,14+,15+,16+,17+,18?,19+,20+,21+,22+,23-,24-,25+,26-,27+,28+,29+,30+,31+,32-,33-,34-,35-,37+/m0/s1 |
|---|
| InChI Key | SXMGGNXBTZBGLU-IBUUTZGMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acylneuraminic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- N-acylneuraminic acid
- Neuraminic acid
- Fatty acyl glycoside
- N-acyl-alpha-hexosamine
- C-glucuronide
- Alkyl glycoside
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Beta-hydroxy aldehyde
- Fatty acyl
- Pyran
- Oxane
- Acetamide
- Alpha-hydroxyaldehyde
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Polyol
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Acetal
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Organonitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Primary alcohol
- Aldehyde
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0400040009-67dd67eda04977a96ef8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01sl-4903063104-47887538c3880ad57980 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08gi-8932240003-1701bae6c829e036f293 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0203-4629130007-71827ae78e1094ab35e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004r-9622220306-e8aa42cc4ca2146a9e89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bvi-9734300000-609eb0e286083d90a1d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006t-7100000029-db42f49a9e486a61b347 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-4160401089-b290fe1b6dbca676e132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000001-0d871c83b17a07c8a848 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0j59-0200011059-ff65ac79c275960b2f4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ea-7504051019-75718b8a0efe28b75c46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fi0-6925030000-d935d2f834b8fc552109 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006593 |
|---|
| FooDB ID | FDB023990 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 58145732 |
|---|
| ChEBI ID | 89905 |
|---|
| PubChem Compound ID | 53477861 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Murakami, Tatsuya; Suzawa, Toshiyuki; Yamazaki, Motoo; Sato, Mitsuo; Endo, Tetsuo; Koizumi, Satoshi; Imada, Teruyoshi. Screening of compounds with specific site affinity, probes for the process, and use of oligosaccharides for drug delivery, diagnostic agents, and pharmaceuticals. Jpn. Kokai Tokkyo Koho (2004), 25 pp. CODEN: JKXXAF JP 2004138397 A 20040513 CAN 140:386061 AN 2004:391539 | | 2. Shen Z, Warren CD, Newburg DS: High-performance capillary electrophoresis of sialylated oligosaccharides of human milk. Anal Biochem. 2000 Mar 1;279(1):37-45. | | 3. Shen Z, Warren CD, Newburg DS: Resolution of structural isomers of sialylated oligosaccharides by capillary electrophoresis. J Chromatogr A. 2001 Jul 6;921(2):315-21. |
|
|---|