| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:47:51 UTC |
|---|
| Update Date | 2016-11-09 01:19:02 UTC |
|---|
| Accession Number | CHEM030293 |
|---|
| Identification |
|---|
| Common Name | Morusin |
|---|
| Class | Small Molecule |
|---|
| Description | An extended flavonoid that is flavone substituted by hydroxy groups at positions 5, 2' and 4', a prenyl group at position 3 and a 2,2-dimethyl pyran group across positions 7 and 8. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
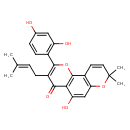
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(2,4-Dihydroxyphenyl)-5-hydroxy-8,8-dimethyl-3-(3-methyl-2-butenyl)-4H,8H-benzo[1,2-b:3,4-b']dipyran-4-one | HMDB | | 2-(2,4-Dihydroxyphenyl)-5-hydroxy-8,8-dimethyl-3-(3-methyl-2-butenyl)-4H,8H-benzo[1,2-b:3,4-b']dipyran-4-one, 9ci | HMDB | | Mulberrochromene | HMDB |
|
|---|
| Chemical Formula | C25H24O6 |
|---|
| Average Molecular Mass | 420.455 g/mol |
|---|
| Monoisotopic Mass | 420.157 g/mol |
|---|
| CAS Registry Number | 62596-29-6 |
|---|
| IUPAC Name | 2-(2,4-dihydroxyphenyl)-5-hydroxy-8,8-dimethyl-3-(3-methylbut-2-en-1-yl)-4H,8H-pyrano[2,3-h]chromen-4-one |
|---|
| Traditional Name | morusin |
|---|
| SMILES | CC(C)=CCC1=C(OC2=C(C(O)=CC3=C2C=CC(C)(C)O3)C1=O)C1=C(O)C=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C25H24O6/c1-13(2)5-7-17-22(29)21-19(28)12-20-16(9-10-25(3,4)31-20)24(21)30-23(17)15-8-6-14(26)11-18(15)27/h5-6,8-12,26-28H,7H2,1-4H3 |
|---|
| InChI Key | XFFOMNJIDRDDLQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-prenylated flavones. These are flavones that features a C5-isoprenoid substituent at the 3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavones |
|---|
| Direct Parent | 3-prenylated flavones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-prenylated flavone
- Pyranoflavonoid
- Hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- Pyranochromene
- 2,2-dimethyl-1-benzopyran
- Chromone
- 1-benzopyran
- Benzopyran
- Resorcinol
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Pyranone
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Pyran
- Benzenoid
- Vinylogous acid
- Heteroaromatic compound
- Oxacycle
- Organoheterocyclic compound
- Ether
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-4228900000-2affcdcba17654d49f18 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-1000039000-abf44c2ad584a39512b9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1004900000-d0374f7defb8b22e4265 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02t9-2009300000-0e88ace1138bfc449492 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066s-6294000000-80140a8ad408ae675a78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0001900000-70e4c74138be78101227 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0007900000-f45325771cf9e098fbb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2947100000-e2f816db44a3bcad85f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-e89f00937a4f746a0b2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000900000-e89f00937a4f746a0b2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-0190100000-4ebaea6ef8aeb8df3495 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-2c722d7f3ff1673a173f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000900000-2c722d7f3ff1673a173f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-0090400000-1353dd108cec26cf250e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036631 |
|---|
| FooDB ID | FDB015550 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001070 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4444990 |
|---|
| ChEBI ID | 7005 |
|---|
| PubChem Compound ID | 5281671 |
|---|
| Kegg Compound ID | C10106 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=25237377 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=25476160 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=25628938 | | 4. Lee JC, Won SJ, Chao CL, Wu FL, Liu HS, Ling P, Lin CN, Su CL: Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem Biophys Res Commun. 2008 Jul 18;372(1):236-42. doi: 10.1016/j.bbrc.2008.05.023. Epub 2008 May 14. | | 5. de Souza MM, Bittar M, Cechinel-Filho V, Yunes RA, Messana I, Delle Monache F, Ferrari F: Antinociceptive properties of morusin, a prenylflavonoid isolated from Morus nigra root bark. Z Naturforsch C. 2000 Mar-Apr;55(3-4):256-60. | | 6. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|