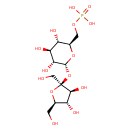| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 21:20:25 UTC |
|---|
| Update Date | 2016-11-09 01:17:47 UTC |
|---|
| Accession Number | CHEM023868 |
|---|
| Identification |
|---|
| Common Name | Sucrose 6-phosphate |
|---|
| Class | Small Molecule |
|---|
| Description | A disaccharide phosphate that is sucrose carrying a single monophosphate substituent at position 6 on the glucose ring. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-O-Phosphonosucrose | ChEBI | | 6-Phosphosucrose | ChEBI | | alpha-D-Glucopyranosyl-(12)-beta-D-fructofuranoside 6-phosphate | ChEBI | | beta-D-Fructofuranosyl-(12)-alpha-D-glucopyranoside 6-phosphate | ChEBI | | beta-D-Fructofuranosyl-6-O-phosphono-alpha-D-glucopyranoside | ChEBI | | Sucrose 6-phosphate | ChEBI | | a-D-Glucopyranosyl-(12)-b-D-fructofuranoside 6-phosphate | Generator | | a-D-Glucopyranosyl-(12)-b-D-fructofuranoside 6-phosphoric acid | Generator | | alpha-D-Glucopyranosyl-(12)-beta-D-fructofuranoside 6-phosphoric acid | Generator | | Α-D-glucopyranosyl-(12)-β-D-fructofuranoside 6-phosphate | Generator | | Α-D-glucopyranosyl-(12)-β-D-fructofuranoside 6-phosphoric acid | Generator | | b-D-Fructofuranosyl-(12)-a-D-glucopyranoside 6-phosphate | Generator | | b-D-Fructofuranosyl-(12)-a-D-glucopyranoside 6-phosphoric acid | Generator | | beta-D-Fructofuranosyl-(12)-alpha-D-glucopyranoside 6-phosphoric acid | Generator | | Β-D-fructofuranosyl-(12)-α-D-glucopyranoside 6-phosphate | Generator | | Β-D-fructofuranosyl-(12)-α-D-glucopyranoside 6-phosphoric acid | Generator | | b-D-Fructofuranosyl-6-O-phosphono-a-D-glucopyranoside | Generator | | Β-D-fructofuranosyl-6-O-phosphono-α-D-glucopyranoside | Generator | | Sucrose 6-phosphoric acid | Generator | | Sucrose-6-phosphoric acid | Generator |
|
|---|
| Chemical Formula | C12H23O14P |
|---|
| Average Molecular Mass | 422.276 g/mol |
|---|
| Monoisotopic Mass | 422.083 g/mol |
|---|
| CAS Registry Number | 22372-29-8 |
|---|
| IUPAC Name | {[(2R,3S,4S,5R,6R)-6-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | sucrose-6-phosphate |
|---|
| SMILES | OC[C@H]1O[C@@](CO)(O[C@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C12H23O14P/c13-1-4-7(16)10(19)12(3-14,25-4)26-11-9(18)8(17)6(15)5(24-11)2-23-27(20,21)22/h4-11,13-19H,1-3H2,(H2,20,21,22)/t4-,5-,6-,7-,8+,9-,10+,11-,12+/m1/s1 |
|---|
| InChI Key | WQQSIXKPRAUZJL-UGDNZRGBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as disaccharide phosphates. These are disaccharides carrying one or more phosphate group on a sugar unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Disaccharide phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Disaccharide phosphate
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Tetrahydrofuran
- Secondary alcohol
- Polyol
- Oxacycle
- Acetal
- Organoheterocyclic compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_16) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0900000000-ce51799a3178a5e28034 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-2960000000-b667587f0dbba369b3a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9700000000-d623b479a3853564fd74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-3910100000-9accf7dcee67c65fd6cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ta-5900000000-77702e31f82d1dcc3254 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9200000000-d232fe0f2095cd4537c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-022a-0903500000-e937b6cc41dd7df6c606 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01pk-3900000000-fd714559fa52e8c17d71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9311000000-cd1c884e237090a4044d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004j-9100100000-e37e29d6ffb40395eae0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9111000000-aa976de33ad26c1a381b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-d058ea1da9c400aa3f07 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0301916 |
|---|
| FooDB ID | FDB001612 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00007452 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-15716 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 559141 |
|---|
| ChEBI ID | 131603 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C16688 |
|---|
| YMDB ID | YMDB16265 |
|---|
| ECMDB ID | ECMDB20196 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|