| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:36:35 UTC |
|---|
| Update Date | 2016-11-09 01:09:17 UTC |
|---|
| Accession Number | CHEM004176 |
|---|
| Identification |
|---|
| Common Name | Paraldehyde |
|---|
| Class | Small Molecule |
|---|
| Description | A trioxane that is 1,3,5-trioxane substituted by methyl groups at positions 2, 4 and 6. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
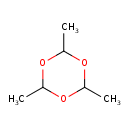
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3,5-Trimethyl-2,4,6-trioxane | ChEBI | | 2,4,6-Trimethyl-S-trioxane | ChEBI | | Acetaldehyde trimer | ChEBI | | Paraacetaldehyde | ChEBI | | Paracetaldehyde | ChEBI | | Paral | ChEBI | | Paraldehyd | ChEBI | | 2,4,6-Trimethyl-1,3,5-trioxaan | HMDB | | 2,4,6-Trimethyl-1,3,5-trioxacyclohexane | HMDB | | 2,4,6-Trimethyl-1,3,5-trioxan | HMDB | | 2,4,6-Trimethyl-1,3,5-trioxane | HMDB | | 2,4,6-Trimetil-1,3,5-triossano | HMDB | | Acetaldehyde, trimer | HMDB | | cis-2,4,6-Trimethyl-1,3,5-trioxane | HMDB | | Elaldehyde | HMDB | | p-Acetaldehyde | HMDB | | Paraldehyde draught (BPC 1973) | HMDB | | Paraldehyde enema (BPC 1973) | HMDB | | Paraldeide | HMDB | | PCHO | HMDB | | S-Trimethyltrioxymethane | HMDB | | S-Trimethyltrioxymethylene | HMDB | | Triacetaldehyde | HMDB | | Trimethyl trioxane | HMDB |
|
|---|
| Chemical Formula | C6H12O3 |
|---|
| Average Molecular Mass | 132.158 g/mol |
|---|
| Monoisotopic Mass | 132.079 g/mol |
|---|
| CAS Registry Number | 123-63-7 |
|---|
| IUPAC Name | 2,4,6-trimethyl-1,3,5-trioxane |
|---|
| Traditional Name | paral |
|---|
| SMILES | CC1OC(C)OC(C)O1 |
|---|
| InChI Identifier | InChI=1S/C6H12O3/c1-4-7-5(2)9-6(3)8-4/h4-6H,1-3H3 |
|---|
| InChI Key | SQYNKIJPMDEDEG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trioxanes. Trioxanes are compounds containing a six-member aliphatic saturated heterocycle made up of three oxygen atoms and three carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Trioxanes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Trioxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3,5-trioxane
- Oxacycle
- Acetal
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0005-9000000000-a3f03c0baa80a3369e84 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0005-9000000000-a3f03c0baa80a3369e84 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-f73aa84f045e831d84cb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1900000000-eb0bbb330df7037330a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-9100000000-7d1ce0bf472280921818 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9000000000-9c5fe2af2a34a0f1f68d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-435a3a910604404881c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-3900000000-297fb8d6656a6904bc17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fu-9000000000-5521d98f9d7d523da88b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006t-9200000000-563a279e2c9ca0c742f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-1648c88bc9d3c0f7a9ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-626ce70022872c1a8363 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a5c-9100000000-bd6564359df9e99046f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9000000000-e713c4a8d1fc500b24d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-889bb2641af78f14431e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB09117 |
|---|
| HMDB ID | HMDB0032456 |
|---|
| FooDB ID | FDB010010 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Paraldehyde |
|---|
| Chemspider ID | 21106173 |
|---|
| ChEBI ID | 27909 |
|---|
| PubChem Compound ID | 31264 |
|---|
| Kegg Compound ID | C07834 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|