| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-19 01:30:44 UTC |
|---|
| Update Date | 2016-10-28 10:01:37 UTC |
|---|
| Accession Number | CHEM003961 |
|---|
| Identification |
|---|
| Common Name | Nitroglycerin |
|---|
| Class | Small Molecule |
|---|
| Description | Nitroglycerin is a vasodilator drug used for the treatment of chest pain and high blood pressure. It was first approved in 2000 and is currently marketed by Pfizer, and other companies, dependiNitroglycerin on the dosage form. Nitroglycerin is available in various forms, includiNitroglycerin a spray form, sublingual tablet form, intravenous form, extended-release tablet form, and transdermal form. A less commonly known fact is that in addition to treatiNitroglycerin angina, nitroglycerin is also used in an ointment to treat the pain that accompanies anal fissures. The rectal ointment form of nitroglycerin was approved by the FDA in 1955. |
|---|
| Contaminant Sources | - Clean Air Act Chemicals
- Cosmetic Chemicals
- EAFUS Chemicals
- HMDB Contaminants - Urine
- HPV EPA Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
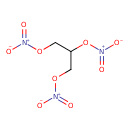
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3-Propanetrioltrinitrate | ChEBI | | 1,2,3-Propanetriyl nitrate | ChEBI | | Glycerin trinitrate | ChEBI | | Glycerol trinitrate | ChEBI | | Glycerol, nitric acid triester | ChEBI | | Glyceryl trinitrate | ChEBI | | Minitran | ChEBI | | Natispray | ChEBI | | NG | ChEBI | | Nitro-dur | ChEBI | | Nitroglycerine | ChEBI | | Nitroglycerol | ChEBI | | Nitrolingual | ChEBI | | Nitromist | ChEBI | | Nitrostat | ChEBI | | Propane-1,2,3-triyl trinitrate | ChEBI | | Rectogesic | ChEBI | | Transderm nitro | ChEBI | | Trinitroglycerin | ChEBI | | Trinitroglycerol | ChEBI | | Nitro-bid | Kegg | | Transderm-nitro | Kegg | | 1,2,3-Propanetrioltrinitric acid | Generator | | 1,2,3-Propanetriyl nitric acid | Generator | | Glycerin trinitric acid | Generator | | Glycerol trinitric acid | Generator | | Glycerol, nitrate triester | Generator | | Glyceryl trinitric acid | Generator | | Propane-1,2,3-triyl trinitric acid | Generator | | Nitroglycerin ointment | HMDB | | NTG | HMDB | | TNG | HMDB | | NitroBid | HMDB | | NitroDur | HMDB | | Nitrocard | HMDB | | Nitroderm | HMDB | | Nitroglyn | HMDB | | Sustak | HMDB | | Gilustenon | HMDB | | Nitro bid | HMDB | | Tridil | HMDB | | Dynamite | HMDB | | Nitrangin | HMDB | | Nitroderm TTS | HMDB | | Nitrolan | HMDB | | Nitrong | HMDB | | Nitrospan | HMDB | | Sustonit | HMDB | | Trinitrate, glyceryl | HMDB | | Trinitrin | HMDB | | Trinitrolong | HMDB | | Anginine | HMDB | | Nitro dur | HMDB | | Nitrol | HMDB | | Perlinganit | HMDB | | Susadrin | HMDB | | Sustac | HMDB |
|
|---|
| Chemical Formula | C3H5N3O9 |
|---|
| Average Molecular Mass | 227.087 g/mol |
|---|
| Monoisotopic Mass | 227.003 g/mol |
|---|
| CAS Registry Number | 55-63-0 |
|---|
| IUPAC Name | 1,3-bis(nitrooxy)propan-2-yl nitrate |
|---|
| Traditional Name | nitroglycerin |
|---|
| SMILES | [O-][N+](=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |
|---|
| InChI Identifier | InChI=1S/C3H5N3O9/c7-4(8)13-1-3(15-6(11)12)2-14-5(9)10/h3H,1-2H2 |
|---|
| InChI Key | SNIOPGDIGTZGOP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl nitrates. These are organic compounds containing a nitrate that is O-linked to an alkyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organic oxoanionic compounds |
|---|
| Sub Class | Organic nitrates |
|---|
| Direct Parent | Alkyl nitrates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl nitrate
- Organic nitro compound
- Organic nitric acid or derivatives
- Organic 1,3-dipolar compound
- Allyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-2910000000-7e0a7efb140cbfba7482 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-7b8724b54d55339884e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0490000000-eccb5cfa311360a6d1cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-7900000000-dfa7cd8533d95e3908a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-3644f8d65b1b573fa474 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-2390000000-d5a66ecca766d2de99bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0v09-2920000000-fe3db65c7d301a910f03 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00727 |
|---|
| HMDB ID | HMDB0014865 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nitroglycerin |
|---|
| Chemspider ID | 4354 |
|---|
| ChEBI ID | 28787 |
|---|
| PubChem Compound ID | 4510 |
|---|
| Kegg Compound ID | C07455 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|