| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-10-14 21:20:43 UTC |
|---|
| Update Date | 2016-11-09 01:09:15 UTC |
|---|
| Accession Number | CHEM003947 |
|---|
| Identification |
|---|
| Common Name | 2-hydroxyflutamide |
|---|
| Class | Small Molecule |
|---|
| Description | 2-hydroxyflutamide is a metabolite of flutamide. Flutamide is an oral nonsteroidal antiandrogen drug primarily used to treat prostate cancer. It competes with testosterone and its powerful metabolite, dihydrotestosterone (DHT) for binding to androgen receptors in the prostate gland. By doing so, it prevents them from stimulating the prostate cancer cells to grow. Flutamide has been largely replaced by a newer member of this class, bicalutamide, due to a better side-effect profile. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Animal Toxin
- Metabolite
- Natural Compound
|
|---|
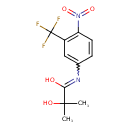
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| OH-Flutamide | HMDB |
|
|---|
| Chemical Formula | C11H11F3N2O4 |
|---|
| Average Molecular Mass | 292.211 g/mol |
|---|
| Monoisotopic Mass | 292.067 g/mol |
|---|
| CAS Registry Number | 52806-53-8 |
|---|
| IUPAC Name | 2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanimidic acid |
|---|
| Traditional Name | hydroxyflutamide |
|---|
| SMILES | CC(C)(O)C(O)=NC1=CC(=C(C=C1)N(=O)=O)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C11H11F3N2O4/c1-10(2,18)9(17)15-6-3-4-8(16(19)20)7(5-6)11(12,13)14/h3-5,18H,1-2H3,(H,15,17) |
|---|
| InChI Key | YPQLFJODEKMJEF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trifluoromethylbenzenes. These are organofluorine compounds that contain a benzene ring substituted with one or more trifluoromethyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Trifluoromethylbenzenes |
|---|
| Direct Parent | Trifluoromethylbenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trifluoromethylbenzene
- Nitrobenzene
- Anilide
- Nitroaromatic compound
- N-arylamide
- Tertiary alcohol
- Carboxamide group
- C-nitro compound
- Secondary carboxylic acid amide
- Organic nitro compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Organic oxoazanium
- Hydrocarbon derivative
- Alkyl halide
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organic oxygen compound
- Organohalogen compound
- Organic nitrogen compound
- Alkyl fluoride
- Organopnictogen compound
- Alcohol
- Carbonyl group
- Organic zwitterion
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 125-130C | | Boiling Point | 443.801 C at 760 mmHg | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9050000000-f84e588ae9034a41b2dc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0089-9611200000-62d9053b443fcb365642 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-06g0-9800000000-18021dcb8f1e955f52ad | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0a6r-6940000000-dd38c2394309e1626e1e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0a6r-6940000000-81afeee01eada15bcf38 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0a4i-2490000000-6d0e498f9c29ac103dd7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0006-0090000000-1892a491307294bb679e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0a4i-0090000000-73482035831e8bedb25c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0a4i-0090000000-e7bc28de00b2591fa92a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-4c17b53efb57846397e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-3090000000-d9e96ed3ee61c4eaf8ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059f-9010000000-67e035c44d2e533ec730 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-c848958ccc3b75da2ac3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1190000000-78cf87749f2c226b1da4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k9i-9200000000-b8932182dc7010369052 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Hydroxyflutamide is an antiandrogen. Antiandrogens alter the androgen pathway by blocking the appropriate receptors, competing for binding sites on the cell's surface, or affecting androgen production. Antiandrogens are classified as steroidal or nonsteroidal. Steroidal antiandrogens not only counter androgens, but also affect secondary sex characteristics. Steroidal antiandrogens directly affect gene expression due to their fat-soluble nature that allows them to diffuse through the plasma membrane's phospholipid bilayer and prevent the binding of testosterone and dihydrotestosterone (DHT) to the androgen receptor. Inhibition of androgen production occurs through a unique mechanism for each antiandrogen. (Wikipedia) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | In men, antiandrogens are most frequently used to treat prostate cancer. In women, antiandrogens are used to decrease levels of male hormones causing symptoms of hyperandrogenism. (Wikipedia) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Environmental antiandrogens can have harmful effects on reproductive organ development in fetuses exposed in utero as well as their offspring. (Wikipedia) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0060949 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Hydroxyflutamide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 91649 |
|---|
| Kegg Compound ID | C14204 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|