| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-17 20:01:34 UTC |
|---|
| Update Date | 2016-11-09 01:09:14 UTC |
|---|
| Accession Number | CHEM003900 |
|---|
| Identification |
|---|
| Common Name | 1-Nitronaphthalene |
|---|
| Class | Small Molecule |
|---|
| Description | 1-nitronaphthalene (1-NN) is a common air pollutant in urban areas. It can react with ozone and will form reactive electrophiles that have been shown to bind covalently to specific proteins. 1-Nitronaphthalene is synthesized by the action of a mixture of nitric and sulfuric acids on finely ground naphthalene. 1-nitronaphthalene is used as a chemical intermediate in the manufacture of dyes (drugs, perfumes, rubber chemicals, tanning agents and pesticides) and as a fluorescence quencher for mineral oils. 1-NN is also found in the gas phase of diesel exhaust, making it one of the more common airborne pollutants. It has been detected in some carbon blacks as well as in particulate exhaust of diesel engines and has been found at low concentrations in ambient air. |
|---|
| Contaminant Sources | - IARC Carcinogens Group 3
- My Exposome Chemicals
- Sludge Chemicals
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Aromatic Hydrocarbon
- Dye
- Industrial Precursor/Intermediate
- Lachrymator
- Organic Compound
- Synthetic Compound
|
|---|
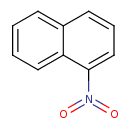
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-Nitronaphthalene | ChEBI | | Nitrol | ChEBI | | a-Nitronaphthalene | Generator | | Α-nitronaphthalene | Generator |
|
|---|
| Chemical Formula | C10H7NO2 |
|---|
| Average Molecular Mass | 173.168 g/mol |
|---|
| Monoisotopic Mass | 173.048 g/mol |
|---|
| CAS Registry Number | 86-57-7 |
|---|
| IUPAC Name | 1-nitronaphthalene |
|---|
| Traditional Name | nitrol |
|---|
| SMILES | O=N(=O)C1=CC=CC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C10H7NO2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H |
|---|
| InChI Key | RJKGJBPXVHTNJL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nitronaphthalenes. These are polycyclic aromatic compounds containing a naphthalene moiety substituted by one or more nitro groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Naphthalenes |
|---|
| Sub Class | Nitronaphthalenes |
|---|
| Direct Parent | Nitronaphthalenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-nitronaphthalene
- Nitroaromatic compound
- Organic nitro compound
- C-nitro compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Metabolic Pathways | Not Available | Not Available | | Homologous recombination | Not Available | map03440 | | Apoptosis | Not Available | map04210 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Yellow needles or green-red to brown crystals. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 61.5°C | | Boiling Point | 304°C | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0fb9-9600000000-bdf73b19a48ec1965ff9 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0fb9-9600000000-bdf73b19a48ec1965ff9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-2900000000-0140e7cd9d0b52504c9a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-33ee06a9c8b5b2d7d4a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-9c34bb8dbc81213babf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gi0-1900000000-b7e76a0a748be5940337 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-b7779eb83652f78c0ed9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-e703c93ccd2f202675fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01b9-1900000000-244213ab51d311f2343b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-e4aa4846c14db133ae00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-e4aa4846c14db133ae00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0900000000-e4aa4846c14db133ae00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-7dd52f7617ae35e45cc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-7dd52f7617ae35e45cc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fb9-7900000000-bc620efb4831b6318040 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-004i-2900000000-4e9a24f4a3738ec10d6f | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation |
|---|
| Mechanism of Toxicity | 1-Nitronaphthalene and its reactive products specifically targets the airway epithelium. Its toxicity is synergized by prior long-term ozone exposure. 1-NN appears to specifically target peroxiredoxin 6 and biliverdin reductase as well as the N-terminal region of calreticulin.
|
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (1) |
|---|
| Uses/Sources | Industrial intermediate, used in production of dyes, found in diesel exhast, air pollutant |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Health effects: May cause eye, skin, respiratory tract and digestive tract irritation. Chronic exposure may cause cancer (based on animal studies). |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0062188 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 34104 |
|---|
| PubChem Compound ID | 6849 |
|---|
| Kegg Compound ID | C14040 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|