| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-09-11 05:23:20 UTC |
|---|
| Update Date | 2016-11-09 01:09:14 UTC |
|---|
| Accession Number | CHEM003896 |
|---|
| Identification |
|---|
| Common Name | alpha-Ionone |
|---|
| Class | Small Molecule |
|---|
| Description | alpha-Ionone belongs to the family of Monocyclic Monoterpenes. These are monoterpenes containing 1 ring in the isoprene chain |
|---|
| Contaminant Sources | - EAFUS Chemicals
- FooDB Chemicals
- HPV EPA Chemicals
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Ester
- Food Toxin
- Household Toxin
- Metabolite
- Organic Compound
- Plant Toxin
- Synthetic Compound
|
|---|
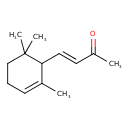
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-alpha-Ionone | ChEBI | | alpha-(e)-Ionone | ChEBI | | alpha-Cyclocitrylideneacetone | ChEBI | | alpha-Ionon | ChEBI | | trans-alpha-Ionone | ChEBI | | (e)-a-Ionone | Generator | | (e)-Α-ionone | Generator | | a-(e)-Ionone | Generator | | Α-(e)-ionone | Generator | | a-Cyclocitrylideneacetone | Generator | | Α-cyclocitrylideneacetone | Generator | | a-Ionon | Generator | | Α-ionon | Generator | | trans-a-Ionone | Generator | | trans-Α-ionone | Generator | | a-Ionone | Generator | | Α-ionone | Generator | | (3E)-4-(2,6,6-Trimethyl-2-cyclohexen-1-yl)-3-buten-2-ON | HMDB | | alpha-Ionone, (e)-isomer | MeSH | | alpha-Ionone, (e)-(+-)-isomer | MeSH | | alpha-Ionone, (+)-iosmer | MeSH |
|
|---|
| Chemical Formula | C13H20O |
|---|
| Average Molecular Mass | 192.297 g/mol |
|---|
| Monoisotopic Mass | 192.151 g/mol |
|---|
| CAS Registry Number | 127-41-3 |
|---|
| IUPAC Name | (3E)-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-one |
|---|
| Traditional Name | α-ionone |
|---|
| SMILES | CC(=O)\C=C\C1C(C)=CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C13H20O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h6-8,12H,5,9H2,1-4H3/b8-7+ |
|---|
| InChI Key | UZFLPKAIBPNNCA-BQYQJAHWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclofarsesane sesquiterpenoid
- Megastigmane sesquiterpenoid
- Sesquiterpenoid
- Ionone derivative
- Alpha,beta-unsaturated ketone
- Enone
- Acryloyl-group
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-4900000000-acff00e4fe614e1e5bc8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0900000000-0d1ee7a07c25e0eb379a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004u-3900000000-122599cd5a3606da3858 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9400000000-5525adff7c75947a3830 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-86ad4191ecec06d667eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-81ca79f706995e1f2313 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aed-2900000000-e754146e5b21f942f91e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0900000000-dca766c12d151f3d74b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fu-8900000000-26ead371c8e940e8859e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9600000000-ad142c26c199a3741a02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-14436e4d9ff8d032db54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-7cd7cf4235cb00fbc0b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kmj-3900000000-b5da866d6db21994e86c | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9800000000-d19fa6227b12fe21dbc3 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0059883 |
|---|
| FooDB ID | FDB014484 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ionone |
|---|
| Chemspider ID | 4445317 |
|---|
| ChEBI ID | 32319 |
|---|
| PubChem Compound ID | 5282108 |
|---|
| Kegg Compound ID | C12286 |
|---|
| YMDB ID | YMDB16094 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|