| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2014-08-29 06:51:46 UTC |
|---|
| Update Date | 2016-11-09 01:09:09 UTC |
|---|
| Accession Number | CHEM003497 |
|---|
| Identification |
|---|
| Common Name | Cyanuric acid |
|---|
| Class | Small Molecule |
|---|
| Description | Because of their trifunctionality, CYA is a precursor to crosslinking agents, especially for polyurethane resins. Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides. In 1997, worldwide production was 160 million kilograms. |
|---|
| Contaminant Sources | - HPV EPA Chemicals
- OECD HPV Chemicals
- STOFF IDENT Compounds
- T3DB toxins
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | - Disinfectant
- Herbicide
- Household Toxin
- Industrial/Workplace Toxin
- Metabolite
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
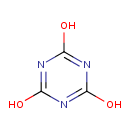
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione | ChEBI | | Isocyanursaeure | ChEBI | | Isozyanursaeure | ChEBI | | S-Triazine-2,4,6-trione | ChEBI | | Cyanate | Generator | | Cyanic acid | Generator | | 1,3,5-Triazin-2,4,6-triol | HMDB | | 1,3,5-Triazine-2,4, 6(1H,3H,5H)-trione | HMDB | | 1,3,5-Triazine-2,4,6-triol | HMDB | | 1,3,5-Triazine-2,4,6-triol (acd/name 4.0) | HMDB | | 2,4,6-Triazinetrione | HMDB | | 2,4,6-Trihydroxy-1,3,5-triazine | HMDB | | 2,4,6-Trihydroxy-S-triazine | HMDB | | 2,4,6-Trihydroxytriazine | HMDB | | 2,4,6-Trioxohexahydro-1,3,5-triazine | HMDB | | 5-Azabarbituric acid | HMDB | | Cyanurate | HMDB | | Cyanursaure | HMDB | | Isocyanurate acid | HMDB | | Isocyanuric acid | HMDB | | Kyselina kyanurova | HMDB | | Pseudocyanuric acid | HMDB | | S-2,4,6-Triazinetriol | HMDB | | S-Triazine-2,4,6(1H,3H,5H)-trione | HMDB | | S-Triazine-2,4,6-triol | HMDB | | S-Triazinetriol | HMDB | | S-Triazinetrione | HMDB | | Sym-triazine-2,4,6-triol | HMDB | | Sym-triazinetriol | HMDB | | Symclosene | HMDB | | Triazine-2,4,6-triol | HMDB | | Tricarbimide | HMDB | | Trichloroisocyanuric acid | HMDB | | Tricyanic acid | HMDB | | Trihydroxycyanidine | HMDB | | Zyanursaure | HMDB | | Cyanuric acid, disodium salt | HMDB | | Cyanuric acid, monosodium salt | HMDB | | Cyanuric acid, sodium salt | HMDB | | Cyanuric acid, trisodium salt | HMDB | | Cyanuric acid, cupric ammonia (+2) salt | HMDB | | Cyanuric acid, potassium salt | HMDB | | Cyanuric acid, monopotassium salt | HMDB | | Isocyanate | HMDB | | Isocyanic acid | HMDB | | Cyanuric acid | MeSH |
|
|---|
| Chemical Formula | C3H3N3O3 |
|---|
| Average Molecular Mass | 129.074 g/mol |
|---|
| Monoisotopic Mass | 129.017 g/mol |
|---|
| CAS Registry Number | 108-80-5 |
|---|
| IUPAC Name | 1,3,5-triazine-2,4,6-triol |
|---|
| Traditional Name | cyanuric acid |
|---|
| SMILES | OC1=NC(O)=NC(O)=N1 |
|---|
| InChI Identifier | InChI=1S/C3H3N3O3/c7-1-4-2(8)6-3(9)5-1/h(H3,4,5,6,7,8,9) |
|---|
| InChI Key | ZFSLODLOARCGLH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3,5-triazines. 1,3,5-triazines are compounds containing a triazine ring, which is a heterocyclic ring, similar to the six-member benzene ring but with three carbons replaced by nitrogen atoms, at ring positions 1, 3, and 5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Triazines |
|---|
| Sub Class | 1,3,5-triazines |
|---|
| Direct Parent | 1,3,5-triazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3,5-triazine
- Heteroaromatic compound
- Azacycle
- Polyol
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 360°C | | Boiling Point | Not Available | | Solubility | 2 mg/mL at 25°C |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-004i-4900000000-61ecfa8dc25736843860 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-004i-4900000000-61ecfa8dc25736843860 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-2900000000-e0493eb157ae373e06af | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00e9-9355000000-d475ee0111dcb61d4778 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-0d99cf3c1a3690dbd6a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-5900000000-fbc75c854286d676b78c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-c99fb76654fd81d51114 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-3409bbee158cd9014a92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-d448f5467302b8fc3082 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-fe35310d6ce849fb2d01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1900000000-ac175bb2b4d3dd57b544 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001u-9600000000-4e031134f5402bad4371 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-6ec906433ed71fbfa134 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-002f-9500000000-a4aa26c95f8fef9c82d4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041861 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CYANURIC-ACID |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cyanuric acid |
|---|
| Chemspider ID | 7668 |
|---|
| ChEBI ID | 17696 |
|---|
| PubChem Compound ID | 7956 |
|---|
| Kegg Compound ID | C06554 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB004758 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | |
|---|